Suggest likely products in the following reactions; (the reactions as shown are not necessarily balanced): (a) xLiI
Question:
Suggest likely products in the following reactions; (the reactions as shown are not necessarily balanced):
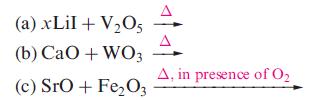
Transcribed Image Text:
(a) xLiI + V₂05 (b) CaO + WO3 (c) SrO + Fe₂O3 A, in presence of 0₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a xLi VO Lithium Vanadate ...View the full answer

Answered By

Ma Kristhia Mae Fuerte
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides): (a) CsF+ XeF4 (b) SiO + XeOF4 (c) XeF + SbF5 (d) XeF6 + [OH] (e) KrF + HO-
-
Suggest products for the following reactions, which are not necessarily balanced on the left-hand side: (a) KrF + Au (b) XeO3 + RbOH 298 K (c) [XeC1] [SbF1] (d) KrF + B (OTeF5)3 (e) C6F-XeF +...
-
Suggest products of the following reactions, which are not necessarily balanced on the left-hand side: (a) AlMe6+ HO (b) AIR3 + R'NH (c) Me3 SiCl + Na[C5H5] (d) Me SiCl + Li [AIH4]
-
Some food retailers propose subjecting food to a low level of radiation in order to improve safety, but sale of such "irradiated" food is opposed by many people. Suppose a grocer wants to find out...
-
Last year, Panacea Laboratories, Inc., researched and perfected a cure for the common cold. Called Cold-Gone, the product sells for $28.00 per package, each of which contains five tablets. Standard...
-
In Exercises 121124, determine whether each statement makes sense or does not make sense, and explain your reasoning. I can use any positive number other than 1 in the change of- base property, but...
-
Identify one organisation that you know and list a set of keywords and a phrase that you think would describe that organisation. Visit the organisations website and note the metatags used for...
-
You were able to purchase two tickets to an upcoming concert for $100 apiece when the concert was first announced three months ago. Recently, you saw that StubHub was listing similar seats for $225...
-
Parallel Inc. has identified the following two mutually exclusive projects: Year Cash Flow (A) Cash Flow (B) 0 $ 38,450 $ 38,450 1 17,900 7,900 2 16,080 13,400 3 12,980 18,800 4 8,880 21,120 a-1....
-
(a) MOCVD with Al(O i Pr) 3 as the precursor can be used to deposit -Al 2 O 3 . Outline the principle of MOCVD, commenting on the required properties of the precursors. (b) Fibres of InN can be grown...
-
(a) The structure of YBa 2 Cu 3 O 7 can be described as consisting of rock salt and perovskite layers. Describe the origin of this description. (b) Why is the potential replacement of NbTi by...
-
Define flood.
-
Find derivative of arcsin x
-
1. Examine the impact of feedwater heater pressure on cycle efficiency and determine the intermediate pressure for optimal performance for one heater.
-
3. Environmental impact of adding feedwater heaters
-
1. Discuss the importance of genetic diversity in fish populations. 2. a) Habitat restoration and connectivity in population conservation is faced with many challenges. Discuss (5 marks) b)a) ...
-
1. Assumethatlengthin Oreochromis variabilis (Victoria Tilapia) ispolygenicvaryingfrom30cmto50cm. A 30cm purebred parent is crossed with another purebred 50 cm individual and the resulting F 1...
-
What concepts of moral philosophy and social responsibility are applicable to the practices of the Canadian automakers described in the introduction to this chapter? Why?
-
Flicker, Inc., a closely held corporation, acquired a passive activity this year. Gross income from operations of the activity was $160,000. Operating expenses, not including depreciation, were...
-
Propose a mechanism for the following reaction. NaH Br
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Calculate S surroundings and S total for part (c) of Problem P5.6. Is the process spontaneous? The state of the surroundings is T = 310.K, P = 0.333 bar.
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


