Suggest products for the following reactions; the lefthand sides of the equations are not necessarily balanced. (a)
Question:
Suggest products for the following reactions; the lefthand sides of the equations are not necessarily balanced.
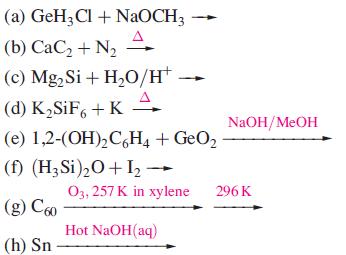
Transcribed Image Text:
(a) GeH₂Cl + NaOCH3 →→ (b) CaC₂ + N₂ (c) Mg₂ Si + H₂O/H™ (d) K₂SiF6+KA (e) 1,2-(OH)₂C6H4 + GeO₂ (f) (H₂Si)₂O+I2₂ →→ (g) €60 (h) Sn NaOH/MeOH O3, 257 K in xylene 296 K Hot NaOH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
GeH3Cl NaOCH3 The reaction is likely a substitution reaction where the chlorine atom in GeH3Cl is replaced by the methoxy group OCH3 from NaOCH3 To fa...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest products for the following reactions; the equations are not necessarily balanced on the lefthand sides. (a) Pl3 + IBr + GaBr3 (b) POBr3 + HF + AsF5 A (c) Pb(NO3)2 liquid NH3 (d) PH3 + K (e)...
-
Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides): (a) CsF+ XeF4 (b) SiO + XeOF4 (c) XeF + SbF5 (d) XeF6 + [OH] (e) KrF + HO-
-
Suggest products for the following reactions; the equations are not necessarily balanced on the left hand sides. Draw the structures of the sulfur containing products. (a) SF4 + SbF5 (b) SO3 + HF....
-
The atomic mass of 14c is 14.003242 u. Show that the decay of 14C is energetically possible, and calculate the energy released in the decay.
-
John Franklin, sole owner of Franklin Mattress Company, has an ownership interest in the company of $50,000 at January 1, 2011. During that year, he invests an additional $10,000 in the company and...
-
Let's assume you do DES double encryption by encrypting with K1 and doing DES in decrypt mode with K2. Does the same attack work as with double encryption with K1 and K2? If not, how could it be made...
-
On January 1,2009, Pierson Corporation exchanged $ 1,710,000 cash for 90 percent ofthe outstand ing voting stock of Steele Company. The consideration transferred by Pierson provided a reasonable...
-
Plain Ltd. acquired 80% of the voting shares of Stylish Ltd. on January 1, 20X3, for $1,200,000. The financial statement of Stylish Ltd. on the date of acquisition was as follows: The inventory will...
-
1.3 Exercise 3 The aggregate demand for a good is the sum over two groups of consumers. Group i's demand is denoted Di, with i = 1,2. The demand functions are respectively equal to Q1 = D.(p) =...
-
What would you expect to be the hydrolysis products of (a) Cyanic acid, (b) Isocyanic acid and (c) Thiocyanic acid?
-
Account for the fact that when aqueous solution of KCN is added to a solution of aluminium sulfate, a precipitate of Al(OH) 3 forms.
-
Rank the time intervals in order of increasing speed. A bicycle is moving along a straight line. The graph shows its position from the starting point as a function of time. Consider the 1 s time...
-
Experiment 3 19. Now click on Circuit 5 and set the values for the Resistors and Voltage as follows: a. R1 =10.0 W, R2 = 20.0 W, R3 = 50.0 W, R4=50.0 W, V=10.0 V. b. Using the formula for adding...
-
This problem is based on the JA Tires data that was first introduced in problem 6-35. If you have not already accessed the data, it can be downloaded from the textbook website or from the attached...
-
Based in Miramichi, New Brunswick, Abenaki Associates Ltd. has been providing information and computer software technology to First Nation customers for more than thirty years. Abenaki Associates is...
-
a = [1,2,3,4] b= a -1 print(b) What is the output? The code does not make errors. Q3. (10 pts) What is the output? The code does not make errors. n=3 for k in range (n) : for m in range (k):...
-
Rodriguez Corporation issues 12,000 shares of its common stock for $62,000 cash on February 20. Prepare journal entries to record this event under each of the following separate situations. 1. The...
-
What is the strategic alliance between HP Hewlett Packard and Hitachi and Samsung, if any?
-
Assume that your audit team has established the following parameters for the examination of ELM's sales transactions: LO G-3 Risk of incorrect acceptance...
-
Explain the important role of cryolite, Na 3 AlF 6 , in the extraction of aluminium metal.
-
How would you expect the Group 13 element to hydrogen bond enthalpy to change as the group is descended? Hence explain the nonexistence of TlH 3 . The most important oxo salts of Group 13 are the...
-
Use suitable molecular-orbital software to calculate the wavefunctions and energy levels for closo-[B 6 H 6 ] 2 . From that output, draw a molecular orbital energy diagram for the orbitals primarily...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


