Suggest products for the following reactions; the equations are not necessarily balanced on the left hand sides.
Question:
Suggest products for the following reactions; the equations are not necessarily balanced on the left hand sides. Draw the structures of the sulfur containing products.
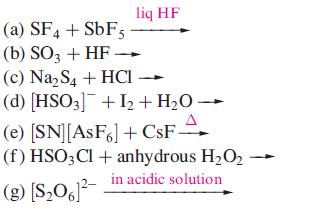
Transcribed Image Text:
(a) SF4 + SbF5 (b) SO3 + HF. liq HF (c) Na₂S4 + HCI (d) [HSO3] + I₂ + H₂O → (e) [SN] [AsF6] + CsF- (f) HSO3Cl + anhydrous H₂O2 in acidic solution (g) [$₂061²-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (7 reviews)
lets look at the products of the given reactions involving sulfurcontaining compounds a...View the full answer

Answered By

Churchil Mino
I have been a tutor for 2 years and have experience working with students of all ages and abilities. I am comfortable working with students one-on-one or in small groups, and am able to adapt my teaching style to meet the needs of each individual. I am patient and supportive, and my goal is to help my students succeed.
I have a strong background in math and science, and have tutored students in these subjects at all levels, from elementary school to college. I have also helped students prepare for standardized tests such as the SAT and ACT. In addition to academic tutoring, I have also worked as a swim coach and a camp counselor, and have experience working with children with special needs.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest products for the following reactions; the equations are not necessarily balanced on the lefthand sides. (a) Pl3 + IBr + GaBr3 (b) POBr3 + HF + AsF5 A (c) Pb(NO3)2 liquid NH3 (d) PH3 + K (e)...
-
Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides): (a) CsF+ XeF4 (b) SiO + XeOF4 (c) XeF + SbF5 (d) XeF6 + [OH] (e) KrF + HO-
-
Suggest products and write balanced equations for each of the following reactions; these are not necessarily balanced on the left-hand side. (a) KOH + HSO4 (b) NaOH + SO (c) KOH + CH5OH (d) Na +...
-
What type of isomers are exhibited by [Fe(en) 3 ]Cl 2 (en = ethane-1,2-diamine)? no isomers are possible. cis and trans isomers fac and mer isomers optical isomers
-
Company P, a U.S. company, has a foreign subsidiary in Country Q, where various forms of bribery are accepted and expected. Company P sent one of its top U.S. managers to oversee operations in its...
-
More info Mar. 1, 2024 Dec. 1, 2024 Dec. 31, 2024 Dec. 31, 2024 Jan. 1, 2025 Feb. 1, 2025 Mar. 1, 2025 Mar. 1, 2025 Borrowed $585,000 from Bartow Bank. The nine-year, 5% note requires payments due...
-
Kayla and Jamie discuss the importance of choosing a career that is balanced between being self-rewarding and also in a field that is sought after in the industry. a. Based on online research,...
-
An analysis of comparative balance sheets, the current years income statement, and the general ledger accounts of Gagliano Corp. uncovered the following items. Assume all items involve cash unless...
-
Miller Manufacturing has a target debtequity ratio of .70. Its cost of equity is 14 percent, and its cost of debt is 7 percent. If the tax rate is 38 percent, what is the companys WACC?
-
(a) How many degrees of vibrational freedom does each of ClF 3 and BF 3 possess? The IR spectrum of ClF 3 in an argon matrix exhibits six absorptions, whereas that of BF 3 has only three. Explain why...
-
(a) Comment on the fact that HOI disproportionates in aqueous solution at pH 0, but in aqueous HCl at pH 0, iodine(I) is stable with respect to disproportionation. (b) The solid state structure of...
-
Why did the Information Systems Audit and Control Association issue their Statements on Auditing Standards?
-
Part 1 of 4 05 points abook Print References Required information Problem 24-2A (Algo) Payback period, accounting rate of return, net present value, and net cash flow calculation LO P1, P2, P3 [The...
-
Keenan Music's CEO has been pondering about the recent proposal of the Specialty Guitar Project. The accountant has done a capital budgeting analysis on the project and outlined the conditions that...
-
On July 1, 2025, Sheridan Co. pays $15,000 to Blue Spruce Insurance Co. for a 2-year insurance contract. Both companies have fiscal years ending December 31. (a1) Journalize the entry on July 1 and...
-
A CU triaxial test with c = 20 psi is performed on a sand and a deviator stress of 80 psi fails the specimen. Previous tests revealed that the effective friction angle for this sand is 35. Calculate...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
During the American Revolution, women faced many challenges while running their households. Often depending on the men of the house only benefited very few, such as women who were married with...
-
Identify the most stable compound:
-
Heating a complex oxide under ammonia can lead to nitridation (with nitrogen replacing oxygen) or reduction (with the production of nitrogen and water). Describe possible products that might be...
-
When NiO is doped with small quantities of Li 2 O, the electronic conductivity of the solid increases. Provide a plausible chemical explanation for this observation.
-
To obtain high oxidation states for first row d metals in complex oxides, e.g. Sr 2 Fe(IV)O 4 , compounds are normally prepared at as low a temperature as possible commensurate with the reaction....
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


