Suggest products for the following reactions (which are not balanced): (a) AgCI+ CIF3 (b) CIF + BF3
Question:
Suggest products for the following reactions (which are not balanced):
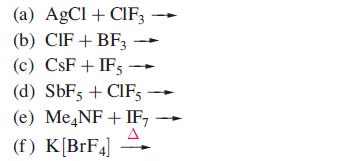
Transcribed Image Text:
(a) AgCI+ CIF3 (b) CIF + BF3 (c) CsF+IFs - (d) SbF5 + CIF5 (e) Me NF + IF₂ A (f) K[BrF4]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a AgCl CIF3 AgF ClF ICl b CI...View the full answer

Answered By

Collins Njuguna
I graduated from Maseno University with a Bachelor of Science in Applied Statistics. After graduation, I started tutoring students in mathematics. My experience in mathematics education is extensive and varied. I have taught a wide range of topics, including algebra, geometry, trigonometry, calculus, statistics, probability, and computer science. I have also worked with students of all ages and backgrounds, from elementary school to college.
My teaching method is based on the idea of hands-on learning. I believe that students learn best when they are actively engaged in the learning process, so I focus on giving students the tools they need to explore the material on their own. I also emphasize the importance of practice and review, as these are essential for mastering math concepts.
I have also developed several online and in-person courses on mathematics. My courses are designed to help students learn mathematics in an efficient and comprehensive way, and I use a variety of activities and exercises to ensure that my students are engaged and motivated.
Overall, my passion for mathematics and teaching has allowed me to be a successful tutor and educator in the field. I am confident that my experience will help your students master the mathematics they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides): (a) CsF+ XeF4 (b) SiO + XeOF4 (c) XeF + SbF5 (d) XeF6 + [OH] (e) KrF + HO-
-
Suggest likely products for the following reactions (which are balanced on the left-hand sides) in liquid NH 3 . How does reaction (d) differ from the behaviour of MeCO 2 H in aqueous solution? (a)...
-
(a) Suggest products for the reaction of Li 3 N with water. Write a balanced equation for the reaction. (b) A compound A was isolated from the reaction between a group 1 metal M and O 2 . A reacts...
-
Figure shows an overhead view of a ring that can rotate about its center like a merry-go-round. Its outer radius R2 is 0.800 m, its inner radius R1 is R2/2.00, its mass M is 8.00 kg, and the mass of...
-
Describe the roles of the following organizations in establishing generally accepted accounting principles: a. The FASB b. The AICPA c. The SEC From which of these organizations can you most easily...
-
On January 1, 20X1, Popular Creek Corporation organized SunTime Company as a subsidiary in Switzerland with an initial investment cost of Swiss francs (SFr) 76,000. SunTime's December 31, 20X1, trial...
-
Comparing Taxes for Employee Benefits. Which of the following employee benefits has the greater value? Use the formula given in the Financial Literacy Calculations feature to compare these benefits....
-
Superior Co. manufactures automobile parts for sale to the major U.S. automakers. Superior's internal audit staff is to review the internal controls over machinery and equipment and make...
-
Requirement 2 please Military Stores is authorized to issue 16,000 shares of common stock. During a two-month period, Military completed these stock transactions: (Click the icon to view the...
-
HSO is an intermediate in the atmospheric oxidation of H 2 S, and it has been implicated in ozone depletion. Calculated wavenumbers for the fundamental modes of vibration of HSO are 2335, 1077 and...
-
The reaction of TeCl 4 with PPh 3 in THF solution in air leads to the formation of the salt (Ph 3 PO) 2 H] 2 [Te 2 Cl 10 ]. Structural data reveal that each Te centre in the anion is in an...
-
A three-phase, eight-pole, 60-Hz, 4160-V, 1250-kW squirrel-cage induction motor has the following equivalent-circuit parameters in ohms-per-phase-Y referred to the stator: R1 = 0.212 R2 = 0.348 X1 =...
-
5.Descibe the HSI color image model 6. Describe the basic relationship between the pixels
-
1. What is the need for transform? 2. What is Image Transform? 3. What are the applications of transform? 4. Give the Conditions for perfect transform . 5. What are the properties of unitary...
-
6. Define Fourier transform pair 7. Define Fourier spectrum and spectral density 8. Give the relation for 1-D discrete Fourier transform pair 9. Specify the properties of 2D Fourier transform. 10....
-
16. What is wrap around error? 17. Give the formula for correlation of 1D continuous function. 18. What are the properties of Haar transform. 19. What are the Properties of Slant transform 20....
-
21. Define fast Walsh transform. 22. Give the relation for 1-D DCT. 23. Write slant transform matrix SN. 24. Define Haar transform. 25. Define K-L transform. 26. Give the equation for singular value...
-
When looking at the application of Social psychology in medicine, what are some things that we can learn from other cultures regarding medicine and the treatment of mental disorders?
-
A bar of a steel alloy that exhibits the stress-strain behavior shown in Figure 6.22 is subjected to a tensile load; the specimen is 375 mm (14.8 in.) long and has a square cross section 5.5 mm (0.22...
-
Carbon nanotubes have been suggested for use as wires in molecular electronics. Describe the challenges of using nanotubes as wires in terms of connecting two functional electronic devices. Describe...
-
Egyptian blue, CaCuSi 4 O 10 , is pale blue and the spinel CuAl 2 O 4 is an intense blue-green. Explain the difference.
-
Discuss how the various physical properties of graphene may lead to the incorporation of this material into future technologies.
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...

Study smarter with the SolutionInn App


