The analysis undertaken in Problem 4.8 may be extended to give the isomegethic rule which states that
Question:
The analysis undertaken in Problem 4.8 may be extended to give the ‘isomegethic rule’ which states that ‘The formula unit volumes, Vm, of isomeric ionic salts are approximately the same’; see H.D.B. Jenkins et al., Inorg. Chem., 2004, 43, 6238, and L. Glasser, J. Chem. Educ., 2011, 88, 581. Discuss the basis for this ‘rule’ and its applications in solid-state chemistry.
Data from Problem 4.8
The following linear correlation, sometimes referred to as Bartlett’s relationship, has been found between lattice enthalpy (kJ mol−1) and the inverse cube root of the formula unit volume V (in nm3) for 1:1 MX salts.
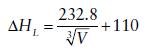
Show that this expression is related to the Kapustinskii equation. Formula unit cell volumes are readily obtainable from X-ray diffraction studies of crystalline, ionic MX structures and, therefore, Bartlett’s relationship may be used where individual thermochemical radii are not available. Discuss how application of this relationship has been used to account for the inability to synthesize [I2]+[AlCl4]− and the instability of [I3]+[AsF6]− (H.D.B. Jenkins, H.K. Roobottom, J. Passmore, and L. Glasser, Inorg. Chem., 1999, 38, 3609).
Step by Step Answer:

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke





