The existence of a maximum oxidation number of +6 for both uranium (Z = 92) and tungsten
Question:
The existence of a maximum oxidation number of +6 for both uranium (Z = 92) and tungsten (Z = 74) prompted the placement of U under W in early periodic tables. When the element after uranium, neptunium (Z = 93), was discovered in 1940, its properties did not correspond to those of rhenium (Z = 75), and this cast doubt on the original placement of uranium. Using standard potential data from Resource section 2, discuss the differences in oxidation state stability between Np and Re.
Data from Resource section 2.
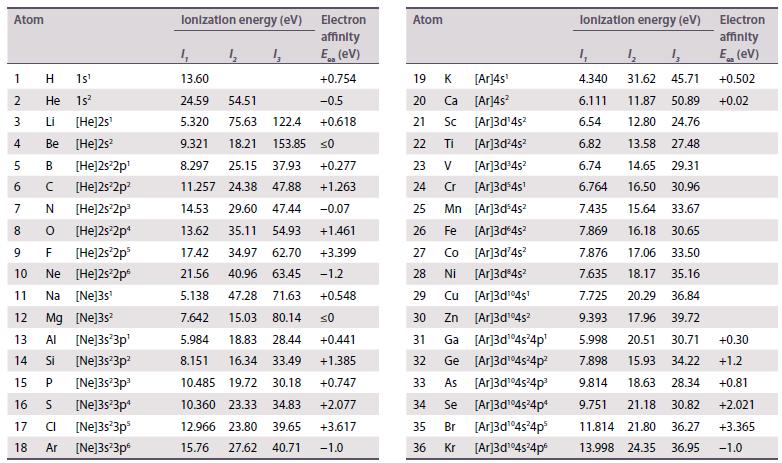
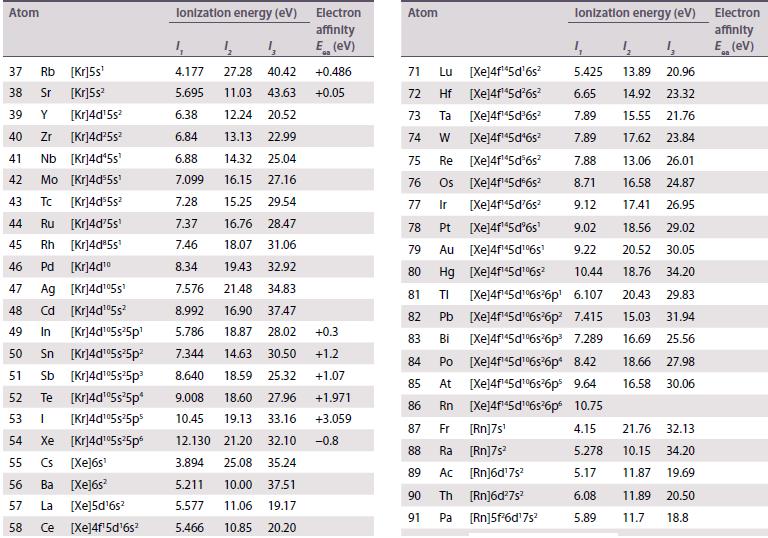
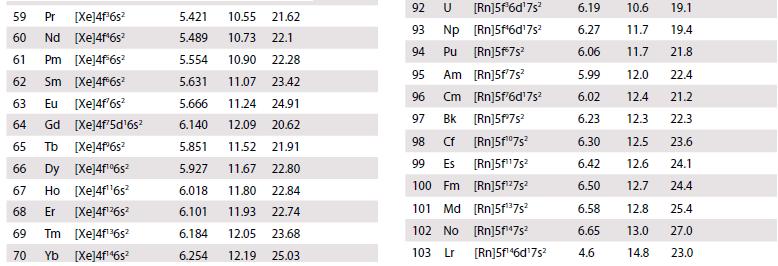
Transcribed Image Text:
Atom 1 H 1s¹ 2 He 1s² 3 Li [He]2s¹ 4 Be [He]2s² 5 B [He]2s²2p¹ 6 C [He]2s²2p² 7 N [He]2s²2p³ 8 0 [He]2s²2p¹ 9 F [He]2s²2p³ 10 Ne [He]2s²2p 11 Na [Ne]3s¹ 12 Mg [Ne]3s² 13 Al [Ne]3s²3p¹ 14 Si [Ne]3s²3p² 15 P [Ne]3s¹3p¹ 16 S [Ne]3s²3p¹ 17 CI [Ne]3s 3ps [Ne]3s²3p6 16 18 Ar Ionization energy (ev) 4 4₂ Electron affinity E (ev) 4 13.60 24.59 54.51 5.320 75.63 122.4 9.321 18.21 153.85 8.297 25.15 37.93 +0.277 11.257 24.38 47.88 +1.263 14.53 29.60 47.44 -0.07 13.62 35.11 54.93 +1.461 17.42 34.97 62.70 +3.399 21.56 40.96 63.45 -1.2 5.138 47.28 71.63 +0.548 7.642 15.03 80.14 ≤0 5.984 18.83 28.44 +0.441 8.151 16.34 33.49 +1.385 10.485 19.72 30.18 +0.747 10.360 23.33 34.83 +2.077 +3.617 -1.0 12.966 23.80 39.65 15.76 27.62 40.71 +0.754 -0.5 +0.618 0 Atom 19 K [Ar]4s¹ 20 Ca [Ar]4s² [Ar]3d¹4s² 21 Sc 22 Ti [Ar]3d²4s² 23 V [Ar]3d³4s² 24 Cr [Ar]3d³4s¹ 25 Mn [Ar]3d³4s² 26 Fe [Ar]3d 4s² 27 Co [Ar]3d²4s² 28 Ni [Ar]3d³4s² 29 Cu [Ar]3d¹04s¹ 30 Zn [Ar]3d¹04s² 31 Ga [Ar]3d¹4s²4p¹ 32 Ge [Ar]3d¹04s²4p² 33 As [Ar]3d¹04s²4p³ 34 Se [Ar]3d¹04s²4pª 35 Br [Ar]3d¹04s²4p³ 36 Kr [Ar]3d¹04s²4p6 Ionization energy (eV) 13 31.62 45.71 11.87 50.89 12.80 24.76 1₁ 4.340 6.111 6.54 6.82 13.58 27.48 6.74 14.65 29.31 6.764 16.50 30.96 7.435 15.64 33.67 7.869 16.18 30.65 7.876 17.06 33.50 7.635 18.17 35.16 7.725 20.29 36.84 9.393 17.96 39.72 5.998 20.51 30.71 7.898 15.93 34.22 9.814 18.63 28.34 9.751 21.18 30.82 11.814 21.80 36.27 13.998 24.35 36.95 Electron affinity E (EV) +0.502 +0.02 +0.30 +1.2 +0.81 +2.021 +3.365 -1.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The differences in oxidation state stability between neptunium Np and rhenium Re can be discussed by considering their electronic configurations posit...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
(8) Consider the series In(n) n=1 (a) Write the first five elements of the sequence of partial sums. (b) Is the sequence of partial sums monotonic, and if so is it non-increasing or non-decreasing?...
-
Lavon Phillips appeals from the district courts entry of summary judgment against him in his Fair Credit Reporting Act * * * claims against his prospective mother-in-law, Mary K. Grendahl; a...
-
This case arose when Kimberly Jasper was terminated from her employment as the director of a child care facility in Johnston, Iowa, called Kid University. The center was owned by H. Nizam, Inc....
-
Rhenium forms a series of solid oxides: Re2O7 (yellow), ReO3 (red), Re2O5 (blue), and ReO2 (brown). One of them has a crystal structure with the following unit cell: a. How many rhenium atoms (gray...
-
Below are some typical transactions incurred by Ricketts Company? 1. Payment of creditors on account. 2. Return of merchandise sold for credit. 3. Collection on account from customers. 4. Sale of...
-
Solution (a) Is 100.0 mL of 0.100 M HCl and solution; (b) Is 150.0 mL of 0.100 M NaCH 3 COO. A few drops of thymol blue indicator are added to each solution. What is the color of each solution? What...
-
Cambi Company began operations on January 1,2008. In the second quarter of 2009, it adopted the FIFO method of inventory valuation. In the past, it used the LIFO method. The companys interim income...
-
Walmart Stores, Inc. (Walmart) is the largest retailing firm in the world. Building on a base of discount stores, Walmart has expanded into warehouse clubs and Supercenters, which sell traditional...
-
Offline anonymous e-money (true digital cash) is the most complex form of e-money because of the double-spending problem. Select one: True or False
-
Discuss the assertion that the chemistry of the actinoids is closer to that of the d-block elements than to that of the lanthanoids.
-
Single molecule magnets (abbreviated SMMs) offer intriguing new possibilities for storing and processing data: lanthanoids, due to their large number of unpaired electrons, are attracting a great...
-
Which employees need to go to training to improve their productivity? is a question that can be answered by: a. Descriptive analytics. b. Diagnostic analytics. c. Predictive analytics. d....
-
Notation Using the weights (Ib) and highway fuel consumption amounts (mi/gal) of the 48 cars listed in Data Set 35 "Car Data" of Appendix B, we get this regression equation: = 58.9 - 0.00749x, where...
-
Week 11-Final Exam: Chapters 5-7 Question 15 of 30 -135 Current At in Ppm 06-20 10%.onthe 1110077 OORE Textbook and M DOLL F T 19 19 Q w A R T Y 3 . 9 4 S D 4 G H A L x N M Cu T
-
We have two samples: sample 1 n= 39 -X= 98.2 S= 15.9 sample 2 n=31 -X=119.2 S= 23.0 begin testing whether u1
-
Discuss charitable purpose trusts under Section 3(1), Charities Act 2011.
-
Amadeus Corporation is considering the issue of a new product to be added to its product mix. They hired you, a recent business graduate from MacEwan, for conducting the analysis. The production line...
-
Suppose that your weekly cash expenses are $80. Every time you withdraw money from the automated teller at your bank, you are charged $.15. Your bank account pays interest of 3% annually. a. How...
-
The following table shows the rates of total return in successive years from 2004 to 2008 for the Sprott Canadian Equity Fund and for the benchmark Toronto Stock Exchange S&P/TSX Composite Index. By...
-
Draw a mechanism for each of the following E1 processes: a. b. c. d. H,SO, Heat Br ETOH, Heat
-
Identify the pattern for each mechanism in Problem 8.34. For example the pattern for Problem 8.34a is: This mechanism is comprised of a proton transfer followed by the two core steps of an E1 process...
-
Identify which of the following methods is more efficient for producing 3,3 dimethylcyclohexene. Explain your choice. Br NaOEt EEOH OH H,SO, Heat
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


