The extraction of metals from primary (naturally occurring ores) and secondary (recycled materials) sources is of huge
Question:
The extraction of metals from primary (naturally occurring ores) and secondary (recycled materials) sources is of huge industrial importance. The manipulation of equilibria in, for example, solvent extraction processes is critically important. The family of bidentate ligands shown below is used to extract metal ions including Cu2+:
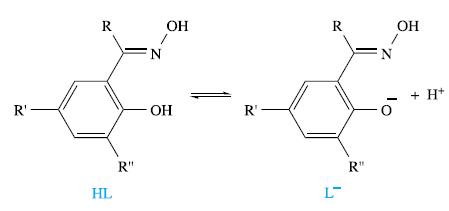
The extraction can be represented by the equilibrium:
![]()
in which ‘org’ refers to extraction into an organic phase.
(a) Why is the extraction process pH dependent?
(b) In [CuL2], the Cu2+ ion is in a square planar environment and the complex can be described as a pseudo-macrocyclic species because of the formation of N–OH · · · O hydrogen bonds. Draw a structure for [CuL2] that is consistent with these observations.
(c) The free ligand, HL, is said to be preorganized towards the formation of [CuL2]. Suggest what this statement means, and comment on relevant equilibria relating to HL in solution.
Step by Step Answer:






