The reaction of [RuCl 2 (PPh 3 )(dppb)] with phen leads to the loss of PPh 3
Question:
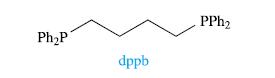
The reaction of [RuCl2(PPh3)(dppb)] with phen leads to the loss of PPh3 and the formation of an octahedral complex, X. The structure of dppb is as follows:
The solution 31P{1H} NMR spectrum of a freshly made sample of X shows a singlet at δ 33.2 ppm. The sample is left standing in the light for a few hours, after which time the 31P{1H} NMR spectrum is again recorded. The signal at δ 33.2 ppm has diminished in intensity, and two doublets at δ 44.7 and 32.4 ppm (relative integrals 1: 1, each signal with J = 31 Hz) have appeared. Rationalize these data.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





