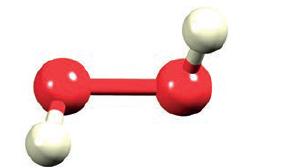
The structure of H 2 O 2 was shown in Fig. 2.1. Apart from the operator E,
Question:
The structure of H2O2 was shown in Fig. 2.1. Apart from the operator E, H2O2 possesses only one other symmetry operator. What is it?
Figure 2.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
H 2 O 2 which is the chemical formula for hydrogen peroxide is a simple molecule with symmetry ...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1. This multipart question deals with an experiment to determine the mass % copper in a mineral sample. The sample does not contain native copper, but instead is a blue and green soft mineral with...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
On November 1, 2021, Sadie's borrows $230,000 from a local bank and signs a note. The note requires interest to be paid annually beginning on August 1, 2022 at 12%. Principal and interest are due in...
-
For the following series of journal entries, indicate whether each is an adjusting entry (ADJ) or a closing entry(CL). SECR. TYPE OF ENTRY ADJ OR CL) ACCOUNTS POST REF. DR. 500 Salaries Expense 500...
-
Simulate the mini-mart situation described in Problem 45 of Chapter 11. Identify the best order quantity using a simulation with 100 trials. Data from Problem 45 of Chapter 11 A gasoline mini-mart...
-
More on bias and reliability. The previous exercise gives 5 true values for lengths. A subject measures each length twice by eye. Make up a set of results from this activity that matches each of the...
-
REPCO performs warranty repair work for name-brand kitchen appliances. REPCO bills appliance manufacturers on a costplus basis. It has a job-costing system that computes the cost of each order by...
-
Costco has an after-tax cost of debt of 9.0%. With a tax rate of 34%, what can you assume the yield is on the debt? Don't round intermediate calculations, and round the final answer to 2 decimal...
-
Which of the following species contain inversion centres? (a) BF 3 ; (b) SiF 4 ; (c) XeF 4 ; (d) PF 5 ; (e) [XeF 5 ] ; (f ) SF 6 ; (g) C 2 F 4 ; (h) HC=C=CH.
-
Volcanoes and deep sea hydrothermal vents are both associated with sulfur-rich environments. Mount Etna is classed as a continuously degassing volcano and emissions of SO 2 and H 2 S are around 1.5...
-
Dual-rate method, budgeted versus actual costs and quantities (continuation of 15-17). Chocolat Inc. decides to examine the effect of using the dual-rate method for allocating truck costs to each...
-
The break even point of a company is $240 000. They sell their product at a markup of 30% and have variable expenses of 9% of sales. They currently make a profit of $10 500. They plan on reducing...
-
What kind of messages are young girls and boys receiving about whether their safety in relationships is valued are by their families and communities? Do we emphasize to our children (boys and girls)...
-
Below are the transactions for Oliver Printing, Incorporated for June, the first month of operations. June 1 Obtain a loan of $ 5 6 , 0 0 0 from the bank by signing a note. June 2 Issue common stock...
-
Assume an organization must invest $ 7 0 0 , 0 0 0 in fixed costs to produce a product that sells for $ 7 5 and requires $ 4 0 in variable costs to produce one unit. What is the organization s...
-
hotel delta marriott montreal What do you think is the value and purpose for the hotel brand choosing to make CSR an important part of their overall business strategy? What two recommendations based...
-
List the disadvantages of using nonparametric statistical procedures?
-
A woman at a point A on the shore of a circular lake with radius 2 mi wants to arrive at the point C diametrically opposite on the other side of the lake in the shortest possible A time. She can walk...
-
For a paramagnetic compound of a d-metal having one unpaired electron, outline the main difference you would expect between an EPR spectrum measured in aqueous solution at room temperature and one...
-
Predict a value for the isomer shift for iron in the Mssbauer spectrum of Na 3 Fe(V)O 4 .
-
The EPR spectrum of bis[tris(2-pyridyl)methane]copper(II) dinitrate is isotropic at 293 K, but is axial with g > g at 150 K. Explain these observations by references to the EPR timescale and...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


