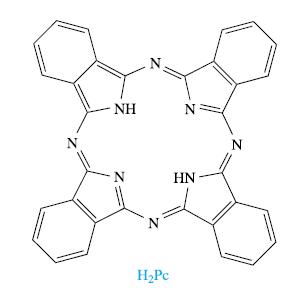
The structure of phthalocyanine is shown below: The complex [Cu(Pc)] is an important commercial pigment and its
Question:
The structure of phthalocyanine is shown below:
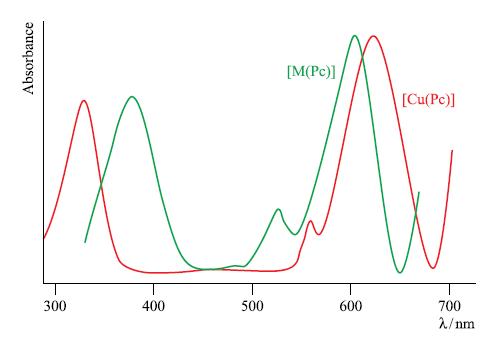
The complex [Cu(Pc)] is an important commercial pigment and its electronic absorption spectrum is shown in Fig. 20.40. The absorption spectrum represented by the green line in Fig. 20.40 arises from another metal(II) phthalocyanine complex, [M(Pc)].
(a) Suggest how the ligand H2Pc binds Cu2+, and draw the structure of [Cu(Pc)]. Comment on its formation in terms of the chelate effect.
(b) What colour is the [Cu(Pc)] pigment?
Rationalize your answer.
(c) The absorptions around 600–650 nm in Fig. 20.40 arise from π* ← π transitions. Explain what this notation means and the origins of the orbitals involved.
(d) Explain how the absorption spectrum of [M(Pc)] gives rise to a green pigment.
(e) When Cu2+ forms a complex with the perchlorinated analogue of H2Pc, there is a red shift in the lowest energy absorption band compared to that in the spectrum of [Cu(Pc)]. How does this affect the colour of the pigment?
(f) Inkjet printing dyes based on [Cu(Pc)] contain sulfonate substituents. Suggest a reason for this.
Figure 20.40.
Step by Step Answer:






