Using the following Latimer diagram, which shows the standard potentials for sulfur species in acid solution (pH
Question:
Using the following Latimer diagram, which shows the standard potentials for sulfur species in acid solution (pH = 0), construct a Frost diagram and calculate the standard potential for the HSO4−/S8(s) couple.
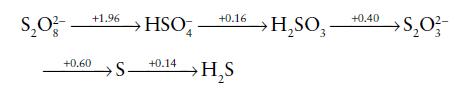
Transcribed Image Text:
$₂0²- +0.60 +1.96 +0.16 HSO +0.14 S- →H₂S → H₂SO3 +0.40 S₂0 ²/3- >
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
To construct a Frost diagram well plot the standard reduction potentials E for each species against ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The following Latimer diagrams show the standard reduction potentials E /V for some oxidation states of iron in acid and alkaline solution: (a) Plot a Frost diagram showing the states of Fe under...
-
The Latimer diagram for vanadium species in acidic (pH = 0) solution is: Using these data: (a) Calculate the potential for the reduction of VO 2+ (aq) to V(s) and write a balanced chemical equation...
-
From the following Latimer diagram (which does not correspond to standard conditions), calculate the value of E for the reaction 2HO 2 (aq) O 2 (g) + H 2 O 2 (aq). Comment on the thermodynamic...
-
Certain merchandising transactions for Yule Park Gifts are listed below. November 2: Purchased 110 souvenir calendars at $9 each from Aloha Co. for cash Nov 4: Paid shipping costs $38 for the...
-
Multiple-Choice Questions 1. Changes in prices of a good causes a. Movement along the demand curve. b. Movement along the supply curve. c. No effect to either curve. d. Both a and b 2. If the market...
-
The kinetics of a homogeneous liquid reaction are studied in a flow reactor, and to approximate plug flow the 48-cm long reactor is packed with 5- mm nonporous pellets. If the conversion is 99% for a...
-
Describe the difference between functional and psychological needs.
-
A partially completed charge sales systems flowchart is shown in Figure 2.37. The flowchart depicts the charge sales activities of the Bottom Manufacturing Corporation. A customers purchase order is...
-
QUESTION 12 The actual costs and the standard costs for direct materials for the manufacture of 2,500 actual units of product are as follows: Actual costs 2,500 pounds at $8.20 per pound Standard...
-
Use Fig. 6.12 to find the approximate potential of an aerated lake at pH = 6. With this information and Latimer diagrams from Resource section 3, predict the species at equilibrium for the elements...
-
Calculate the equilibrium constant of the reaction from the standard potentials Aut(aq) + 2 CN- (aq) [Au(CN)](aq)
-
Identify at least three typical company policies that restrict e-mail sent or received by employees while on the employers premises.
-
Section Three Answer the questions below 1.While pulling out of her driveway, Bethany becomes distracted by a bee and strikes Melanie, who is riding past on a bicycle. Bethany suffers serious injury...
-
A __________ is a schedule periodic check of a specific process behavior. Question 1Answer A. Widget B. Dashboard C. Monitor D. Process ID
-
1. Was VAAF contractually obligated to pay Chad for refraining from smoking? 2. Was there consideration to support its promise to pay $500? 3. Are there other facts you need to know to make that...
-
Presented here are the comparative balance sheets of Hames Incorporated at December 31, 2023 and 2022. Sales for the year ended December 31, 2023, totaled $1,700,000.%0D%0A%0D%0AHAMES...
-
McDonald's conducts operations worldwide and is managed in two primary geographic segments: US, and International Operated Markets, which is comprised of Australia, Canada, France, Germany, Italy,...
-
Suppose P(A) = . 4, P(B) = .7, and P(A B) = .3. Find the following probabilities: a. P(B c ) b. P(A c ) c. P(A U B)
-
You've been asked to take over leadership of a group of paralegals that once had a reputation for being a tight-knit, supportive team, but you quickly figure out that this team is in danger of...
-
(a) Explain how single-walled carbon nanotubes are classified in zigzag and armchair tubes. (b) What inherent properties of single-walled carbon nanotubes make those formed by arc discharge or laser...
-
The properties of graphene nanoribbons depend upon their edge structure. What are the different edge structures and how do they arise?
-
Graphene is insoluble in common solvents. Describe ways in which the material can be solubilized.
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


