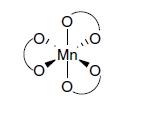
Which isomer is the following tris(acac) complex? O, I Mn. O
Question:
Which isomer is the following tris(acac) complex?
Transcribed Image Text:
O, I Mn. O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The image you sent is not of a trisacac complex ...View the full answer

Answered By

User l_998468
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Suffocation of naphthalene is reversible at elevated temperature. A different isomer of naphthalenesulfonic acid is the major product at 160C than is the case at 0C. Which isomer is the product of...
-
Sulfonation of naphthalene is reversible at elevated temperature. A different isomer of naphthalenesulfonic acid is the major product at 160C than is the case at 0C. Which isomer is the product of...
-
One of the following two stereoisomers is 20 kJ/mol (4.9 kcal/mol) less stable than the other. Indicate which isomer is the less stable, and identify the reason for its decreased stability.
-
Write a critical review paper on the topic of financial management in the broad sense.
-
Moe Green estimates the cost of future projects for a large contracting firm. Mr. Green uses precisely the same techniques to estimate the costs of every potential job, and formulates bids by adding...
-
Technology is the tool that enables people to perform. Managers must have the understanding and skills of how to make the best use of technology, and knowledge of likely new technologies that could...
-
How could the problems of interpretation mentioned above be overcome?
-
Lookhere.Com and StopIn.Com enter into a reciprocal agreement whereby (1) StopIn.Com is given valuable advertising space on the home page of Lookhere.Com and (2) Lookhere.Com is given valuable...
-
The Sarbanes-Oxley Act (SOX) was passed in 2002. The legislation was intended to prevent accounting fraud. What did offending companies do to cause legislators to get involved in the situation? What...
-
The two compounds [RuBr(NH 3 ) 5 ]Cl and [RuCl(NH 3 ) 5 ]Br are what types of isomers?
-
Draw the structures of representative complexes that contain the ligands (a) en, (b) ox 2 , (c) phen, (d) 12-crown-4, (e) tren, (f) terpy, (g) edta 4 .
-
Curtis Hendrix orally agreed to compensate Beverly Spertell for services rendered in connection with their living together out of wedlock. Later, when suit was brought to collect the money, Hendrix...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
Identify the type of continuous random variable-uniform, normal, or exponential-described by each of the following probability density functions: a. f(x) = (e - x/7)2/7, x > 0 b. f(x) = 1/20, 5 c....
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
(a) Analyse the catalytic cycle shown in Fig. 25.19, identifying the types of reactions occurring. (b) Why does this process work best for R' = vinyl, benzyl or aryl groups? Figure 25.19. Pd(OAc) R'X...
-
Ruthenium(II) complexes of the general type shown below are potential anticancer drugs: The cytotoxicity of such complexes relies upon the replacement of the chlorido ligand by H 2 O, and is pH...
-
Suggest products in the following reactions: (a) Excess FeCl 3 with ( 5 -Cp) 2 Fe; (b) ( 5 -Cp) 2 Fe with PhC(O)Cl in the presence of AlCl 3 ; (c) ( 5 -Cp) 2 Fe with toluene in the presence of Al and...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


