Which of the following reactions are redox reactions? In those that are, identify the oxidation and reduction
Question:
Which of the following reactions are redox reactions? In those that are, identify the oxidation and reduction processes.
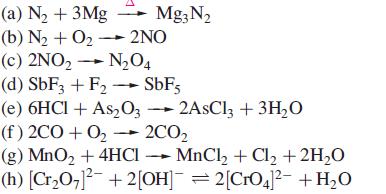
Transcribed Image Text:
(a) N₂ + 3Mg Mg3N₂ (b) N₂ + O₂- 2NO (c) 2NO₂ → N₂04 (d) SbF3 + F₂ → SbF5 (e) 6HCl + As2O32AsCl3 + 3H₂O (f) 2CO+O₂ → 2CO₂ (g) MnO₂ + 4HCI MnCl₂ + Cl₂ + 2H₂O (h) [Cr₂O7]²¯ + 2[OH]¯ = 2[CrO4]²¯ +H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To determine whether each of the given reactions is a redox reductionoxidation reaction we need to check if there are changes in oxidation states for the elements involved In a redox reaction at least ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each redox reaction in problem 8.3, confirm that the net increases and decreases in oxidation states balance each other. Data from Problem 8.3 Which of the following reactions are redox reactions?...
-
The commercial production of nitric acid involves the following chemical reactions: (a) Which of these reactions are redox reactions? (b) In each redox reaction identify the element undergoing...
-
Which of the following reactions involves neither oxidation nor reduction? A) N2 + 3H2 ? 2NH3 B) NH4NO2 ? N2 + 2H2O C) Cu + 2Ag+ ? Cu2+ + 2Ag D) 2CrO42- + 2H+ ? Cr2O72- + H2O E) C2H4 + H2 ? C2H6 I...
-
rewrite/downside Integrity and credibility are the ethics of professional practice that Juan Gomez was lacking in this instance. Juan Gomez lacked integrity because he created a conflict of interest...
-
The structure of international trade and tariff systems is highly complex. To maintain order, the United Nations Conference on Trade and Development (UNCTAD) has developed a coding system that...
-
In Exercises test for convergence or divergence and identify the test used. n=0 5 718 L n
-
How does your university add value to students through the implementation of the four Ps?
-
DeSoto Tools Inc. is planning to expand production. The expansion will cost $300,000, which can be financed either by bonds at an interest rate of 14 percent or by selling 10,000 shares of common...
-
As a result of President Trumps tariffs, a recent survey of executives at Global 1000 companies uncovered what single biggest threat? a. the fear of the unknown b. the fear of improperly weighting...
-
What oxidation state change does each metal undergo in the following reactions or half-reactions? (a) [CrO7- + 14H+ +6e2Cr+ (b) 2K + 2HO2KOH + H (c) FeO3 + 2A1 2Fe + AlO3 A (d) [MnO4] + 2HO + 3e MnO...
-
(a) Calculate E Ag + /Ag for a half-cell in which the concentration of silver(I) ions is 0.1moldm 3 (T = 298 K). (b) Are silver(I) ions more or less easily reduced by zinc in this solution than under...
-
General Journal Entries for Materials Consider the following data compiled by the manufacturer of 1,000 television tables: Standard price per square foot $.65 Actual price per square foot $.62...
-
How has face book influenced political candidate's electoral success? What is the relationship between social media technology called face book and electoral success?
-
1. Discuss the international strategies that organizations can pursue 2. Identify and compare the various modes of of foreign market entry 3. Analyse the industry market 4. Evaluate relevant macro...
-
A bulk carrier was underway. The vessel was in ballast and hold washing was scheduled in preparation for taking the next cargo. An officer, bosun, and another deck crew conducted a risk assessment...
-
8. Neutrino radiation was observed over a certain period and the number of hours in which 0, 1, 2,... signals were received was recorded. 0 1 Number of Number of Hours with Signals per Hour This...
-
What are some advantages and disadvantages of centralization and decentralization. References: Altamimi, H., Liu, Q., & Jimenez, B. (2023). Not Too Much, Not Too Little: Centralization,...
-
Does the location of your seat in a classroom play a role in attendance or grade? To answer this question, professors randomly assigned 400 students* in a general education physics course to one of...
-
Create a data model for one of the processes in the end-of-chapter Exercises for Chapter 4. Explain how you would balance the data model and process model.
-
Describe the delafossite structure type adopted by some compounds of the stoichiometry MMX 2 . Which elemental combinations are known to form this structure type? Discuss their electronic properties...
-
Use ionic radii data (Resource section 1) to suggest possible dopants to increase anion conductivity in (a) PbF 2 (b) Bi 2 O 3 (six-coordinate Bi 3+ ). Resource section 1. Ionic radii are given (in...
-
The compound Fe x O generally has x < 1. Describe the probable metal ion defect that leads to x being less than 1.
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


