Question: As an engineer in a production facility, your assignment is to specify the size of a reactor needed to react a liquid stream(33 L/min) containing
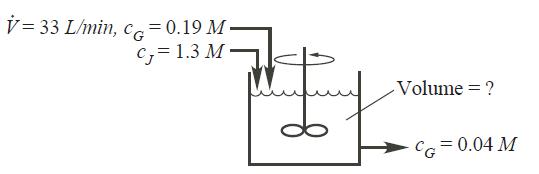
As an engineer in a production facility, your assignment is to specify the size of a reactor needed to react a liquid stream(33 L/min) containing species G (concentration = 0.19 M). The goal is to produce a reactor outlet stream with a concentration of G equal to 0.04 M. To accomplish that, a second stream containing species J (concentration = 1.3 M) is also to enter the reactor but at 75% of the volumetric flow rate of the first stream, as shown.
The irreversible reaction is G + J →P where the reaction rate only depends on species G according to the following kinetic relation:
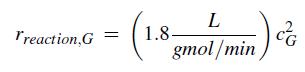
Given these requirements, what size reactor (L) is needed to produce these results? (Assume equal densities for all streams.)
V=33 L/min, CG= 0.19 M C, 1.3 M fuf Volume = ? CG= 0.04 M
Step by Step Solution
3.26 Rating (164 Votes )
There are 3 Steps involved in it
To size the reactor we need to carry out a material balance for species G We will use the design equation for a continuous stirredtank reactor CSTR Fo... View full answer

Get step-by-step solutions from verified subject matter experts


