(a) Prove (b) For an ideal gas along an adiabat, (P/P i ) = (T/ T i...
Question:
(a) Prove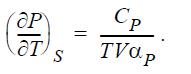
(b) For an ideal gas along an adiabat, (P/Pi) = (T/ Ti)CP/R. Demonstrate that this equation is consistent with the expression from part (a).
Transcribed Image Text:
JP OT S || P TVOp
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), and volume V (in liters) is PV = nRT, where is the number of moles of the gas and R = 0.0821 is the...
-
The specific heat at constant pressure for an ideal gas is given by cp = 0.9 + (2.7 x 10-4) T (kJ/kg K) where T is in kelvin. The change in the enthalpy for this ideal gas undergoing a process in...
-
The isentropic process for an ideal gas is expressed as Pv k = constant. Using this process equation and the definition of the speed of sound (Eq. 179), obtain the expression for the speed of sound...
-
Kiki purchases a $16,000 bond from Green Corp. on January 1, 2021. The bond has a 10% annual interest rate. On December 1, 2021, Kiki gifts the bond to her son, Branson. How much income is reported...
-
Draw a supply-and-demand diagram to explain the effect of a negative externality that occurs as a result of a firms production process.
-
Even though the labor attorney had not yet filed a lawsuit with the courts, was it wise of the company to terminate each of the general managers? Why or why not? L01
-
Understand standardization and adaptation of international marketing. LO.1
-
1. How does the information technology development for video-based businesses differ from traditional businesses? 2. What challenges do these types of companies face in relation to the rapid changes...
-
Bq incorporated is considering making an iffer to purchase ireport publications. The vice president of finance has collected the following infomation. Price earning ration Bq 1 4 . 8 ireport 9 . 2 ,...
-
Determine the difference C P - C V for the following liquids using the data provided near 20C. Liquid (a) Acetone (b) Ethanol (c) Benzene (d) Carbon disulfide (e) Chloroform (f) Ethyl ether (g)...
-
Express the Joule-Thomson coefficient in terms of measurable properties for the following: (a) Van der Waals equation given in Example 6.6 (b) An ideal gas. Example 6.6 Accounting for T and Vimpacts...
-
Prove that the 1-norm and the sup-norm also satisfy Theorem 8.6.
-
Coaching for Performance Develop a strategy for how you will approach the coaching session with the employee, including what you plan to discuss and any questions you may have when you debrief....
-
For the following exercises, find the derivatives of the given functions: 1. y=x-secx+1 2. y = 3 cscx+ 5 3. f(x) = x cotx 4. f(x) = secx I 5. y=
-
1. why does Amazon use ERP system? How does ERP system work for Amazon? what are the benefit and drawbacks of using ERP for Amazon? 2. what are 5 industry best practices across Finance,...
-
A rigid vessel contains afuel gasconsisting of a methane (CH4) and ethane (C2H6) mixture. The pressure in the vessel is found to be 0.30 bar.Air is added to the vessel until the total pressure...
-
Do you agree with this discussion post? My article discusses decision-making tools in Project Management (PM). "In research and development (R&D), project management (PM) decision-making tools are...
-
The following information is provided for Kelly Plumbing Supply. Cash received from customers during December 2017................$387,000 Cash paid to suppliers for inventory during December...
-
Parkin Industries, a U.S. company, acquired a wholly-owned subsidiary, located in Italy, at the beginning of the current year, for 200,000. The subsidiary's functional currency is the euro. The...
-
Using activities, find [Ag + ] in 0.060 M KSCN saturated with AgSCN(s).
-
Using activities, calculate the pH and concentration of H + in 0.050 M LiBr at 25 C.
-
Using activities, calculate the pH and concentration of H + in 0.050 M LiBr at 25C.
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


