Calculate the equilibrium constant for the vapour phase hydration of ethylene at (145^{circ} mathrm{C}). The following data
Question:
Calculate the equilibrium constant for the vapour phase hydration of ethylene at \(145^{\circ} \mathrm{C}\). The following data are given:
\[ \frac{C_{P}}{R}=A+B T+C T^{2}+\frac{D}{T^{2}} \]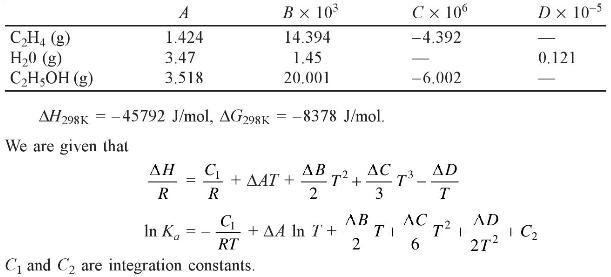
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





