For a pressure-explicit equation of state, prove that the Joule/Thomson inversion curve is the locus of states
Question:
For a pressure-explicit equation of state, prove that the Joule/Thomson inversion curve is the locus of states for which: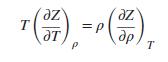 Apply this equation to (a) the van der Waals equation; (b) the Redlich/Kwong equation. Discuss the results.
Apply this equation to (a) the van der Waals equation; (b) the Redlich/Kwong equation. Discuss the results.
Transcribed Image Text:
ze dp T ze T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
The JouleThomson inversion curve is the locus of states for which the temperature change during an i...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Take nitrogen to be a van der Waals gas with a = 1.352 dm6 atm mol-2 and b = 0.0387 dm3 mol-1, and calculate Hm when the pressure on the gas is decreased from 500 atm to 1.00 atm at 300 K. For a van...
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
A certain gas obeys the van der Waals equation with a =0.76 m6 Pa mol-2, its volume is found to be 4.00 X 10-4 m3 mol-1 at 288 K and 4.0 MPa. From this information calculate the van der Waals...
-
Passenger table (passengerid, address etc.) Flight table (flight id, departure, destination, depDate) Booking table (cID, fid, date, cost) a. Find Passengers who live in Chicago b. Total number of...
-
Describe three guidelines for writing an effective specific purpose statement for your informative presentation.
-
When Lehman was developing its Repo 105 accounting policy, did E&Y have a responsibility to be involved in that process? In general, what role should an audit firm have when a client develops an...
-
Find out whether your university provides you with access to SPSS. If it does, visit this books companion website and download the self-teach package and associated data sets. Work through this to...
-
The adjusted trial balance for Anara Co. as of December 31, 2013, follows. P. Anara invested $40,000 cash in the business during year 2013 (the December 31, 2012, credit balance of the P. Anara,...
-
Do journal entry one and two
-
Which tutors, by name, are available to tutor? Write the SQL command.
-
A fan is (in effect) a gas compressor which moves large volumes of air at low pressure across small (1 to 15 kPa) pressure differences. The usual design equation is: where subscript 1 denotes inlet...
-
For isentropic expansion in a converging/diverging nozzle with negligible entrance velocity, sketch graphs of mass flow rate m, velocity u, and area ratio A/A 1 versus the pressure ratio P/P 1 ....
-
Show that in 3D the translation operators \(P_{j}\) and rotation operators \(L_{j}\) given by Eq. (12.9) generate the non-abelian Lie group \(\mathrm{E}_{3}\), with the commutators (12.10). Data from...
-
For this online discussion, we will explore the relevance of various management styles in the context of your respective organizations. Your task is to review different management styles and propose...
-
Is a t-Distribution Appropriate? A sample with size n = 10 has x = 508.5, and s = 21.5. The dotplot for this sample is given below. 0000 00 500 510 520 530 540 550 560 570 Indicate whether or not it...
-
Interpret the results. Write a statement to summarize your conclusion. Is a relationship present? Do we accept or reject the null hypothesis? Are the two variables related? Why or why not?
-
Case study information Australian Renewable Energy Hub Source: https://research.csiro.au/hyresource/australian-renewable-energy-hub/ April 20th, 2023 The Australian Renewable Energy Hub (AREH) will...
-
Listening is a crucial leadership skill that is essential for building effective relationships and solving problems. Write a paper that explores the importance of listening as a leadership skill,...
-
Carpenter Company adopted a defined benefit pension plan for its employees on January 1, 2019. At the time of adoption, the pension contract provided for retroactive benefits for the companys active...
-
Problem 3.5 (4 points). We will prove, in steps, that rank (L) = rank(LT) for any LE Rnxm (a) Prove that rank (L) = rank (LTL). (Hint: use Problem 3.4.) (b) Use part (a) to deduce that that rank(L) =...
-
For one of the substances in Table 3.2, compute the change in volume and work done when one kilogram of the substance is heated from 15C to 25C at a constant pressure of 1 bar. Table 3.2 Table 3.2:...
-
Various species of hagfish, or slime eels, live on the ocean floor, where they burrow inside other fish, eating them from the inside out and secreting copious amounts of slime. Their skins are widely...
-
The Tait equation for liquids is written for an isotherm as: where V is molar or specific volume, V 0 is the hypothetical molar or specific volume at zero pressure, and A and B are positive...
-
The payroll register of Ruggerio Co. indicates $13,800 of social security withheld and $3,450 of Medicare tax withheld on total salaries of $230,000 for the period. Federal withholding for the period...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...

Study smarter with the SolutionInn App


