For a ternary solution at constant T and P, the composition dependence of molar property M is
Question:
For a ternary solution at constant T and P, the composition dependence of molar property M is given by:
M = x1 M1 + x2 M2 + x3 M3 + x1 x2 x3C
where M1, M2, and M3 are the values of M for pure species 1, 2, and 3, and C is a parameter independent of composition. Determine expressions for M̅1, M̅2, and M̅3 by application of Eq. (10.7). As a partial check on your results, verify that they satisfy the summability relation, Eq. (10.11). For this correlating equation, what are the M̅i at infinite dilution?
Eq. (10.7)
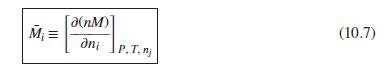
Eq. (10.11)

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





