Sodium fluoride, NaF, is dissolved in water at an apparent concentration of C B = 10 -3
Question:
Sodium fluoride, NaF, is dissolved in water at an apparent concentration of CB = 10-3 mol/L. Construct a Sillèn diagram and estimate the pH. Refer to the pKa,A and pKa,B values in Table 18.2.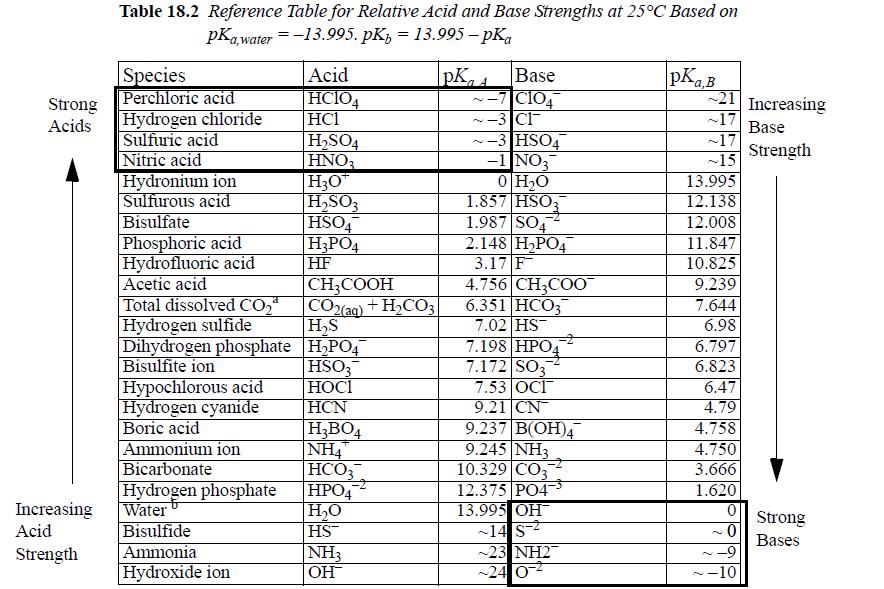
Transcribed Image Text:
Strong Acids Increasing Acid Strength Table 18.2 Reference Table for Relative Acid and Base Strengths at 25°C Based on pKa,water = -13.995. pKz 13.995-pka Species Perchloric acid Hydrogen chloride Sulfuric acid Nitric acid Hydronium ion Sulfurous acid Bisulfate Phosphoric acid Hydrofluoric acid Acetic acid Total dissolved CO₂ Hydrogen sulfide Dihydrogen phosphate Bisulfite ion Hypochlorous acid Hydrogen cyanide Boric acid Ammonium ion Bicarbonate Hydrogen phosphate Water Bisulfide Ammonia Hydroxide ion Acid HCIO4 HC1 H₂SO4 HNO3 H3O™ H₂SO3 HSO4 H3PO4 HF CH3COOH CO2(aq) + H₂CO3 H₂S H₂PO47 HSO3 НОСI HCN H₂BO4 |NH4 HCO3 HPO4 H₂O HS™ NH3 OH PKA Base ~-7 C104 ~-3 Cl -3 HSO4 -1 NO3 0 H₂O 1.857 HSO3 1.987 SO4 2.148 H₂PO4 3.17 F 4.756 CH3COO 6.351 HCO3 7.02 HS 7.198 HPO4 7.172 SO3 7.53 OCI 9.21 CN 9.237 B(OH)4 9.245 NH3 10.329 CO3 12.375 PO4³ 13.995 OH™ -14 S-2 ~23 NH2 -24 0² pKa,B 21 Increasing ~17 Base Strength ~17 ~15 13.995 12.138 12.008 11.847 10.825 9.239 7.644 6.98 6.797 6.823 6.47 4.79 4.758 4.750 3.666 1.620 0 ~0 -9 -10 N Strong Bases
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
Constructing a Silln diagram also known as a Bjerrum plot or a distribution diagram involves plotting the fraction of various species as a function of ...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
A large company is looking to acquire a smaller business. The company believes that, under their management, free cash flows for upcoming years should be forecasted as shown below, and would continue...
-
( ( i ) ) An abstract Use Case is one that is: A . . connected to an Actor but not initiated by another Use Case B . . connected to an Actor and initiated by another Use Case C . . not connected to...
-
Let Find all values of k for which: (a) A has eigenvalues 3 and -1. (b) A has an eigenvalue with algebraic multiplicity 2. (c) A has no real eigenvalues. k 2
-
What is the formula to find total dividend and payout ratio? This is the information I have: the amount of shares the company holds and the last dividend paid. Lastly, will there be enough cash to...
-
Explain the two types of problems and decisions. Contrast the three decision-making conditions.
-
Describe the characteristics and differences between qualitative, quantitative, extrinsic, and intrinsic forecasting techniques.
-
7. Assume that a subsidiary has 10,000 shares of stock outstanding, of which 8,000 shares are owned by the parent. What equity method adjustment will be necessary on the parent books if the...
-
Wes acquired a mineral interest during the year for $10 million. A geological survey estimated that 250,000 tons of the mineral remained in the deposit. During the year, 80,000 tons were mined, and...
-
1: A manufacturing company that incurs shipping costs to deliver its product to a customer; would be classify this cost as a(n): a) Direct materials b) Administrative expense c) Manufacturing...
-
Calcium chloride is used occasionally as an alternative to sodium chloride for de-icing walkways. It is rumored to maintain puddles even a day or so after all evidence of sodium chloride has...
-
(a) Compute the freezing point depression for an aqueous solutions that is 3 wt% NaCl. (b) Compute the boiling point elevation for an aqueous solutions that is 3 wt% NaCl. (c) Compute the osmotic...
-
The comparative financial statements of ODohertys Irish Grille for 2016, 2015, and 2014 include the following selected data: Requirements 1. Compute these ratios for 2016 and 2015: a. Quick ratio b....
-
Write out the form of the partial fraction decomposition of the function (See Example). Do not determine the numerical values of the coefficients. (If the partial fraction decomposition does not...
-
Listed here are the costs associated with the production of 1,000 drum sets manufactured by TrueBeat. Costs 1. Plastic for casing$16,000 2. Wages of assembly workers$83,000 3. Property taxes on...
-
The diagram shows the instant when a long slender bar of mass 4.8 kg and length 2.9 m is horizontal. At this instant the mass m= 6.2 kg has a vertical velocity of 5.3 m/s. If the pulley has...
-
Holland has been down to the Law Clinic and has returned with several new cases/clients; Wanda and the doorman from the 7-Seas Bar/Grill,Wanda, who has been charged with malicious destruction of a...
-
2) A spring of constant k=1.2 N/cm is being compressed 5-cm by a 200-gram ball. The ball is released on a 10 degree incline. Determine: a) The speed of the ball when it leaves the spring b) The...
-
A study on the enhancing effect of coffee on long-term memory found that 35 participants given 200 mg of caffeine performed better on a memory test 24 hours later compared to the placebo group that...
-
How can you tell from the vertex form y = a(x - h) 2 + k whether a quadratic function has no real zeros?
-
A 0.100 M solution of the weak acid HA was titrated with 0.100 M NaOH. The pH measured when V b = V e was 4.62. Using activity coefficients, calculate pKa. The size of the A - anion is 450 pm.
-
Finding the end point from pH measurements. Here are data points around the second apparent end point in Figure 10-5: (a) Prepare a spreadsheet or table analogous to Figure 10-6, showing the first...
-
Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is 9.25 at a volume of 10.00 mL. (a) Suppose you used the yellow-to-blue transition of thymol...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


