Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is
Question:
Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is 9.25 at a volume of 10.00 mL.
(a) Suppose you used the yellow-to-blue transition of thymol blue indicator to find the end point. According to Table 10-3, the last trace of green disappears near pH 9.6. What volume of base is required to reach pH 9.6? The difference between this volume and 10 mL is the indicator error.
(b) If you used cresol red, with a color change at pH 8.8, what would be the indicator error?
Figure 10-2
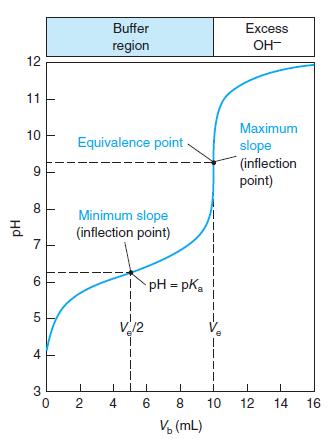
Table 10-2
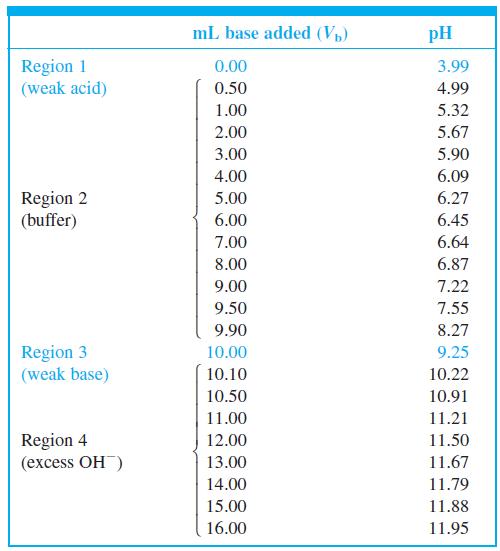
Buffer Excess region OH- 12 11 Maximum 10 Equivalence point slope (inflection point) Minimum slope (inflection point) 6. pH = pK, Ve/2 4 4 6 8. 10 12 14 16 V, (mL) 2. 7,
Step by Step Answer:
a The volume of base required to reach pH 96 can be calculated using the HendersonHasselbalch equati...View the full answer



Related Video
A substance that alters color in solution over a constrained range of pH values is known as a pH indicator or acid-base indicator. The indicator chemical just needs to alter color slightly in order to be noticed. Indicators work on the basis that they react with water to produce the hydrogen cation H+ or hydronium ion H3O+. The indicator molecule\\\'s color changes as a result of the reaction. Since indicators have distinct color ranges for color change, they can occasionally be combined to provide color changes over a wider pH range.
Students also viewed these Engineering questions
-
Consider the titration of 25.0 mL of 0.010 0 M Sn 2+ by 0.050 0 M Tl 3+ in 1 M HCl, using Pt and saturated calomel electrodes to find the end point. (a) Write a balanced titration reaction. (b) Write...
-
Consider the titration in Figure 10-2, for which the pH at the equivalence point is calculated to be 9.25. If thymol blue is used as an indicator, what color will be observed through most of the...
-
Consider the titration of 100.0 mL of 0.010 0 M Ce 4+ in 1 M HClO 4 by 0.040 0 M Cu+ to give Ce 3+ and Cu 2+ , using Pt and saturated Ag | AgCl electrodes to find the end point. (a) Write a balanced...
-
Suppose a wagon moves due east at 10.1 m/s while a skateboard heads pi 3 radians south of east at 12 m/s. What are the x- and y- components of the velocity of the wagon relative to the skateboard?
-
Does the research question stay the same throughout the research process? Why or why not?
-
Why are the maximax and maximin strategies considered to be optimistic and pessimistic, respectively? L01
-
What is the net impact on Werners net income for the quarter ended March 31, 2009, as a result of this forward contract hedge of a firm commitment? LO9 a. $-0-. b. $ 1,250 increase in net income. c....
-
A company has a bonus plan that states that managers with division income ranked below the average of all managers receive no bonus for the year. What biases might arise in this system?
-
Write up the asset and liability and capital accounts to record the following transactions in the records of G. Smith and balance off each account. (4 pts each) June 8 Sold some of the office...
-
Ajax Investment Company is considering the purchase of land that could be developed into a class A office project. At the present time, Ajax believes that the site could support a 300,000 rentable...
-
Finding the end point from pH measurements. Here are data points around the second apparent end point in Figure 10-5: (a) Prepare a spreadsheet or table analogous to Figure 10-6, showing the first...
-
Spectrophotometry with indicators. Acid-base indicators are themselves acids or bases. Consider an indicator, HIn, which dissociates according to the equation The molar absorptivity, , is 2 080 M -1...
-
Postretirement benefits other than pensions (OPRBs) are similar to defined benefit pension plans in some respects and different in others. Required: a. Discuss the characteristics of OPRBs that make...
-
The force vector F has a magnitude of F = 385 lb and acts at point A at an angle 0 = 17 with respect to vertical as shown. The force F is balanced by the tension forces parallel to the two rods AC...
-
D1 Justify the use of a specific moulding technique for the manufacture of a given product
-
the igniter is made of a wire with paper tape holding it . In the head of the igniter is a very thin wire surrounded by pyrotechnic material. Pressing the second switch allows more current to flow...
-
Problem - Process Costing Atticus Electronics produces travel batter pack chargers. The company uses a process costing system. The following information pertains to operations for November Percentage...
-
B . what is the wavespeed? C . What is the frequency? D . What is the wave number? E . At t = 0 . 4 9 s , what is the diplacement of the string at x = 5 . 2 m ?
-
Refer to the Heat Transfer Engineering (Vol. 34, 2013) study of bubble behavior in subcooled flow boiling, Exercises 11.6 and 11.29. You fit an interaction model for bubble density (y) as a function...
-
The process of collaborative goal setting by a manager and subordinate, the extent to which goals are accomplished is a major factor in evaluating and rewarding the subordinate's performance. It is...
-
Would tris(2,2 -bipyridine)iron be a useful indicator for the titration of Sn 2+ with Mn(EDTA) - ? (Hint: The potential at the equivalence point must be between the potentials for each redox couple.)
-
Explain what we mean by preoxidation and prereduction. Why is it important to be able to destroy the reagents used for these purposes?
-
Write balanced reactions for the destruction of S 2 O 2 -8 , Ag 2+, and H 2 O 2 by boiling.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


