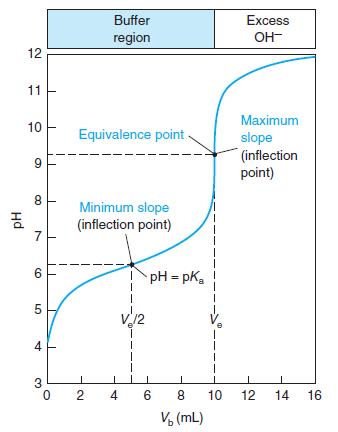
Consider the titration in Figure 10-2, for which the pH at the equivalence point is calculated to
Question:
Consider the titration in Figure 10-2, for which the pH at the equivalence point is calculated to be 9.25. If thymol blue is used as an indicator, what color will be observed through most of the titration prior to the equivalence point? At the equivalence point? After the equivalence point?
Figure 10-2
Transcribed Image Text:
Buffer Excess region OH- 12 11 Maximum 10 Equivalence point slope (inflection point) 9 Minimum slope (inflection point) 7 6 pH = pK, 12 3 2 4 8. 10 12 14 16 V, (mL) 4. Hd
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
First lets see the pH transition range for thymol blue 12red 28yellow 80yellow 96blu...View the full answer

Answered By

Pulkit Sahu
1. I have 3 years of tutoring experience in Chemistry subject.
2. 6 years of Industrial Experience in the field of oil and gas.
3. I like to solve problems in Chemistry.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider the titration of 100.0 mL of 0.10 M phosphoric acid with 0.10 M NaOH. a. Determine the pH at the third half- equivalence point by assuming it is a special point (see Fig.). b. Calculate the...
-
Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is 9.25 at a volume of 10.00 mL. (a) Suppose you used the yellow-to-blue transition of thymol...
-
Consider the titration of 50.0 mL of 0.100 M sodium glycinate, H2NCH2CO2Na, with 0.100 M HCl. (a) Calculate the pH at the second equivalence point. (b) Show that our approximate method of...
-
Enviro-Tech has only two retail and two wholesale customers. Information relating to each customer for 2012 follows (in thousands): Enviro-Tech's annual distribution-channel costs are $33 million for...
-
What standards must you abide by when signing a return or recommending a tax return position to a taxpayer? What must you do to satisfy this standard?
-
Robert Klassen Manufacturing, a medical equipment manufacturer, subjected 100 heart pacemakers to 5,000 hours of testing. Halfway through the testing, 5 pacemakers failed. What was the failure rate...
-
On September 30, 2009, Ericson Company negotiated a two-year, 1,000,000 dudek loan from a foreign bank at an interest rate of 2 percent per year. It makes interest payments annually on September 30...
-
Julia Dumars is a licensed CPA. During the first month of operations of her business, Julia Dumars, Inc., the following events and transactions occurred. May 1 Stockholders invested $20,000 cash in...
-
Holcomb Corporation owns machinery with a book value of $190,000. The machinery's fair value less costs to sell is $175,000, and its value-in-use is $200,000. Holcomb should recognize a loss on...
-
Consecutive five-year balance sheets and income statements of Laura Gibson Corporation are shown below. Operating lease payments were as follows: 2009, $30,000; 2008, $27,000; 2007, $28,500; 2006,...
-
Write the formula of a compound with a negative pK a .
-
What color do you expect to observe for cresol purple indicator (Table 10-3) at the following pH values? (a) 1.0; (b) 2.0; (c) 3.0 Table 10-3 Acid color Transition Base Indicator range (pH) color...
-
Explain why FVA can be calculated for a transaction without considering the portfolio to which the transaction belongs, but that the same is not true of MVA.
-
Test the given claim. Assume that a simple random sample is selected from a normally distributed population. Use either the P-value method or the traditional method of testing hypotheses. Company A...
-
Trojan Technologies As Joyce Guo, senior buyer at Trojan Technologies Inc. in London, Ontario, Canada, finished her presentation, Randy Haill, materials manager, Made the following comments to her:...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
Express the confidence interval (0.045,0.123) in the form of p^ - E < p < p^+ E.
-
Boomtown is preparing a cost analysis of the three departments: Parks. Fire, and Water. To comply with accuracy standards in allocating indirect costs, Boomtown will employ the step-down method of...
-
J. Vuorinen carried out a series of experiments to gather information on the coefficient of permeability of concrete (Magazine of Concrete Research, Sept. 1985). In one experiment, the outflow of...
-
Suppose the spot and six-month forward rates on the Norwegian krone are Kr 5.78 and Kr 5.86, respectively. The annual risk-free rate in the United States is 3.8 percent, and the annual risk-free rate...
-
(a) A 1.5-mm-diameter steel sphere (7830 kg/m 3 ) is dropped into a tank of SAE 30 oil. What is its terminal velocity? (b) If the sphere is instead dropped into a different oil of the same density...
-
A 175-lb skydiver reaches a terminal velocity of 150 mph during free fall. If the frontal area of the diver is 8 ft 2 , what are: (a) The magnitude of the drag force acting on the skydiver? (b) The...
-
A 14-mm-diameter sphere is dropped into a beaker of SAE 30 oil. Over a portion of its downward motion, the sphere is measured to fall at 2 m/s. In the units of newtons, what is the drag force acting...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


