An ideal gas undergoes an adiabatic, reversible expansion process in a closed system: (a) If cp is
Question:
An ideal gas undergoes an adiabatic, reversible expansion process in a closed system:
(a) If cp is constant, show that: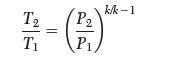
(b) Determine the relationship between temperatures and pressures for an ideal gas if the heat capacity is given by:![]()
Transcribed Image Text:
T2 T || P P. k/k-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The compressor discussed in Illustrations 3.4-4 and 4.5-1 is being used to compress air from 1 bar and 290 K to 10 bar. The compression can be assumed to be adiabatic, and the compressed air is found...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Maria owns and runs her own online premium clothing business (sole trader) named "Maria's Clothing Solutions (MS). Her main competitor is "Brenda's Extraordinary Clothing (BC). She is interested in...
-
Explain how the bankruptcy of Lehman Brothers (a large securities firm) reduced the liquidity of the commercial paper market.
-
The rolling department of Krause steel company had 4800 tons in beginning work in progress inventory (80% complete) on October 1. During October, 80,000 times were completed. The ending work and...
-
1. The spot price of a widget is \($70.00\) per unit. Forward prices for 3, 6, 9, and 12 months are \($70.70\), \($71.41\), \($72.13\), and \($72.86\). Assuming a 5% continuously compounded annual...
-
Consider the following key performance indicators and classify each indicator according to the balanced scorecard perspective it addresses. Choose from the financial perspective, customer...
-
Andrew is considering buying a new taming tractor for his farm. He has a choice between a John Deere XP tractor and a Sunflower-FT tractor. Andrew has a MARR of 8%. John-Deere-XP: First Cost S150,000...
-
Methane vapor enters a valve at 3 bar and 25C and leaves at 1 bar. If the methane undergoes a throttling process, what is the exit temperature, in C? Under these conditions, you may assume methane is...
-
You wish to measure the temperature and pressure of steam fl owing in a pipe. To do this task, you connect a well-insulated tank of volume 0.4 m3 to this pipe through a valve. This tank initially is...
-
A financial feasibility study is being carried out for a proposed new 120- seat restaurant. It will be open for both lunch and dinner from Monday through Saturday and for dinner only on Sunday. For...
-
Discuss the Competitive Markets and Externalities simulations (both with and without policy interventions) . What impact do policy interventions have on the supply and demand equilibrium for a...
-
The best consultant to fix issue number one is Frederick Taylor who is credited with creating the scientific management movement (Lumen, n.d.). Since Taylor's work focused on how a process could be...
-
1. Which Pepsico products are growing faster than soft drinks (why) and by what percentage? 2. Why do the fastest growing products experience a more complex supply chain? Explain. 3. What are some of...
-
Use BLUF (Bottom Line UP Front) or Brief for answering the following questions: 1) There are a number of InfoSec frameworks / models available in industry. A. What is an InfoSec framework / model? B....
-
An introduction to organizational structure. Topics such as alternative organizational structures, the reciprocal relationship between multinational strategy and structure, and how recourses affect...
-
Suppose you need to download a file that is 400,000 bytes in size. Assume that control characters involved in the download increase the total number of downloaded bytes by 10% (to 440,000 bytes)....
-
For the next several days, take notes on your listening performance during at least a half-dozen situations in class, during social activities, and at work, if applicable. Referring to the traits of...
-
Select the compound in each of the following pairs that will be converted to the corresponding alkyl bromide more rapidly on being treated with hydrogen bromide. Explain the reason for your choice....
-
Assuming that the rate-determining step in the reaction of cyclohexanol with hydrogen bromide to give cyclohexyl bromide is unimolecular, write an equation for this step. Use curved arrows to show...
-
Assuming that the rate-determining step in the reaction of 1-hexanol with hydrogen bromide to give 1-bromohexane is an attack by a nucleophile on an alkyloxonium ion; write an equation for this step....
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


