Calculate the enthalpy of mixing for HCl from the enthalpy of solution data reported in Table 6.1.
Question:
Calculate the enthalpy of mixing for HCl from the enthalpy of solution data reported in Table 6.1.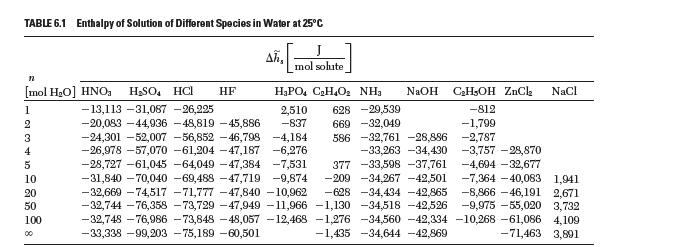
Transcribed Image Text:
TABLE 6.1 Enthalpy of Solution of Different Species in Water at 25C Ah, J mol solute n NaOH CH5OH ZnCl NaCl HPO4 CH4O NH 2,510 628 -29,539 -812 669 -32,049 -1,799 -2,787 586 -32,761 -28,886 -33,263 -34,430 [mol HO] HNO HSO4 HC -13,113 -31,087 -26,225 -20,083-44,936 -48,819 -45,886 -837 -24,301 -52,007 -56,852 -46,798 -4,184 -26,978 -57,070 -61,204 -47,187 -6,276 -3,757 -28,870 -28,727 -61,045 -64,049 -47,384 -7,531 377 -33,598 -37,761 -4,694 -32,677 -31,840 -70,040 -69,488 -47,719 -9,874 -209 -34,267 -42,501 -7,364-40,083 1,941 -32,669 -74,517 -71,777 -47,840 -10,962 -628 -34,434 -42,865 -8,866-46,191 2,671 -32,744 -76,358 -73,729 -47,949 -11,966 -1,130 -34,518 -42,526 -9,975 -55,020 3,732 -32,748 -76,986 -73,848 -48,057 -12,468 -1,276 -34,560 -42,334 -10,268 -61,086 4,109 -33,338 -99,203 -75,189 -60,501 -1,435 -34,644 -42,869 -71,463 3,801 1 2 3 4 5 10 20 50 100 00 HF
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
In a process at your plant, you are mixing two liquids: benzene (1) and 2-propanol (2). You would like to create a molar enthalpy vs. composition diagram for this mixture and have found molar...
-
Use the data of Table 13.1 to calculate the enthalpy of solution of LiCl. Data given in Table 13.1 TABLE 13.1 Data for Calculating Enthalpy of Solution Compound LiF NaF KF RbF LICI NaCl KCI RbCl NaOH...
-
An experiment is performed to measure the enthalpy of mixing of chloroform, CHCl3, and acetone, C3H6O. In this experiment, pure species inlet streams of different compositions are mixed together in...
-
Contact local employers and ask for copies of their employee handbooks. If none are available, research parent companies of local employers online to see if their employee handbooks are available....
-
Based on the case : Polaris & Victory: Entering and Growing the Motorcycle Business Explain: 1- External Environment 2- General Environment 3- Menneto knew his bikes were good, but had they been...
-
Customer profitability in a manufacturing firm. Bizzan Manufacturing makes a component they call P14-31. This component is manufactured only when ordered by a customer, so Bizzan keeps no inventory...
-
LO23 Compute the total cost and the unit product cost of a job using a plantwide predetermined overhead rate.
-
Prepare a comprehensive report for Mario in which you outline and analyze the possible revenue models that ASIB might use for its Web site. You should address the two journals as separate issues....
-
Fogerty Company makes two products-titanium Hubs and Sprockets. Data regarding the two products follow. Direct Labor- Hours per Annual Unit Production 0.90 12,000 units 0.50 47,000 units Hubs...
-
What is the heat requirement to dilute an inlet aqueous stream of 50% NaOH, by weight, to a fi nal concentration of 10%?
-
How does the enthalpy of mixing data for H2SO4 given by Equation (6.24) compare to the enthalpy of solution data from Table 6.1? Is the agreement reasonable? What are the reasons they may be...
-
In Exercises 3134, write the first five terms of each geometric sequence. a 1 = 1/2, r = 1/2
-
3. Different positions of Vermiform appendix.
-
Define Image?
-
What is Dynamic Range?
-
Define Brightness?
-
What is Chromatic Adoption?
-
Say that you have inherited a sterling silver necklace with a six-carat ruby pendant from a distant relative and you would like to sell it on eBay. What would you take into account when considering...
-
C- Consider the following scenario:- A supermarket needs to develop the following software to encourage regular customers. For this, the customer needs to supply his/her residence address, telephone...
-
Assign the following molecules to point groups: (a) HF, (b) IF7 (pentagonal bipyramid), (c) XeO2F2, (see-saw), (d) Fe,(CO)9 (22), (e) Cubane, C8H8, (f) Tetrafluorocubane, C8H4F4 (23) cO Fe co 23
-
Which of the molecules in Exercises 12.9b and 12.10b can be? (a) Polar, (b) Chiral?
-
Consider the C3Yion NO;. Is there any orbital of the central N atom that can have a nonzero overlap with the combination 2pz (A) pz (B) pz (C) of the three O atoms (with z perpendicular to the...
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


