Consider a binary mixture of species 1 and 2 that obeys the PengRobinson equation of state with
Question:
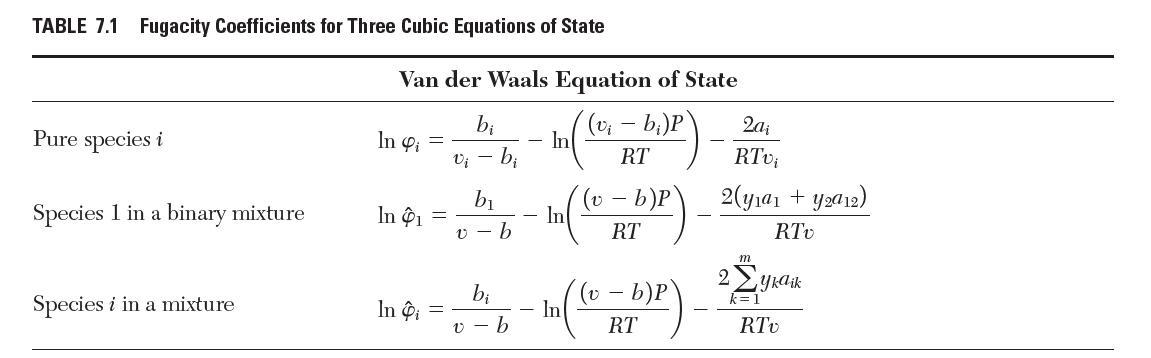
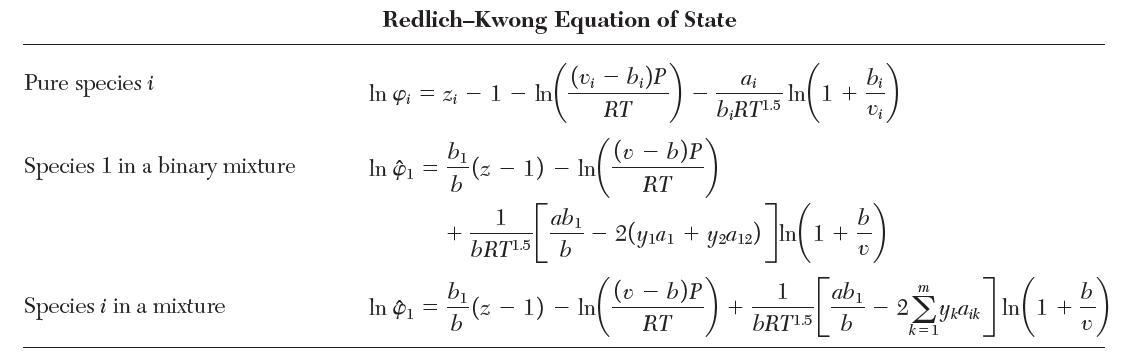
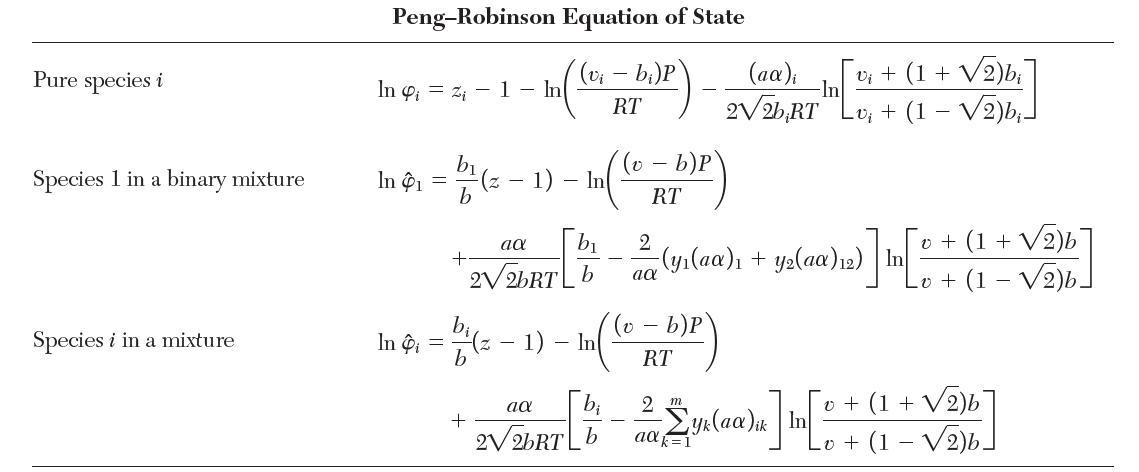
Consider a binary mixture of species 1 and 2 that obeys the Peng–Robinson equation of state with van der Waals mixing rules. Show that the fugacity coeffi cient for species 1 is given by the “Species 1 in a binary mixture” row in Table 7.1.
Transcribed Image Text:
TABLE 7.1 Fugacity Coefficients for Three Cubic Equations of State Pure species i Species 1 in a binary mixture Species i in a mixture Van der Waals Equation of State (v - b)P RT In 4 = In 1 In Pi - bi v - b b b b v-b - - In In In (v b)P RT - b)P RT 2a RTvi 2(ya + ya12) RTV 2 m yka ik RTv
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
An organ pipe is open at one end and closed at the other. The lowest note you can play on this pipe has frequency fmin. If the speed of sound in the pipe is v, what is the length L of the pipe? A L V...
-
Consider a binary mixture of species 1 and 2 that obeys the RedlichKwong equation of state with van der Waals mixing rules. Show that the fugacity coeffi cient for species 1 is given by the Species 1...
-
Example 8.6 illustrates how you solve a dew-point calculation for a binary mixture of a nonideal liquid and a nonideal gas with T known. This problem corresponds to quadrant I in Figure 8.2. Develop...
-
Understand the content theories of motivation.
-
What were the threats for the InfoSys before they entered the "Building Tomorrow's Enterprise" phase? List opportunities that existed on a market for the InfoSys back then? Was the industry...
-
In some countries the market for long-term corporate debt is limited, and firms turn to short-term bank loans to finance long-term investments in plant and machinery. When a short-term loan comes...
-
Goal approach to assessing organizational effectiveness Systems resource approach Participant-satisfaction model Human resource approach Stakeholder approach Competing values approach LO.1
-
Calculating Annuity Values If you deposit $3,000 at the end of each of the next 20 years into an account paying 10.5 percent interest, how much money will you have in the account in 20 years how much...
-
You are trying to develop a strategy for investing in two different stocks. The anticipated annual return for a $1,000 investment in each stock under four different economic conditions has the...
-
Calculate the fugacity and the fugacity coeffi cient of phenol in a mixture of 20 mole % phenol (1) and 80 mole % oxygen (2) at 694.2 K and 24.52 bar using the following: (a) The ideal gas law (b)...
-
Consider a ternary system of methane (a), ethane (b), and propane (c) at 25C and 15 bar. Assume this system can be represented by the virial equation truncated at the second term: At 25C, the second...
-
Figure 16 displays a scatterplot that compares the mean lengths and mean heights of 51 types of dinosaurs. Let L be the mean length and H be the mean height, both in meters, of a dinosaur. a....
-
Companies that invest heavily in eco-friendly initiatives, such as transitioning to renewable energy sources or implementing carbon offset programs, may initially face increased operational costs....
-
Answer each question individually please. 14-13 What are the advantages and drawbacks of universities using social media to communicate with various stakeholdersstudents, potential students, alumni,...
-
act as a consultant hired by the operations director of the Barry Computer Company provide a financial analysis and comparison to the industry. You will conduct a financial ratio analysis to gain a...
-
Building a sense of community is not just a moral thing to do, but also a pragmatic one. In today's competitive and ever-evolving business environment, the organizations that can attract the most...
-
Watch https://youtu.be/U3MtvvNjUR4 What do you think of Dr. Saint's ideas about barriers to change? What do you think about social learning? Could this tool be used to make real change? How can the...
-
Give an example of a change in the marketing environment that would have important implications for managing a products pricing.
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
The equilibrium constant for the reaction N2 (g) + O,(g) ;:='02NO(g) is 1.69 x 10-3 at 2300 K. A mixture consisting of 5.0 g of nitrogen and 2.0 g of oxygen in a container of volume 1.0 dm3 is heated...
-
What is the standard enthalpy of a reaction for which the equilibrium constant is (a) doubled, (b) halved when the temperature is increased by 15 K
-
The dissociation vapour pressure ofNH4Cl at 427C is 608 kPa but at 459C it has risen to 1115 kPa. Calculate (a) The equilibrium constant, (b) The standard reaction Gibbs energy, (c) The standard...
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...

Study smarter with the SolutionInn App


