Consider a binary mixture of species 1 and 2 that obeys the RedlichKwong equation of state with
Question:
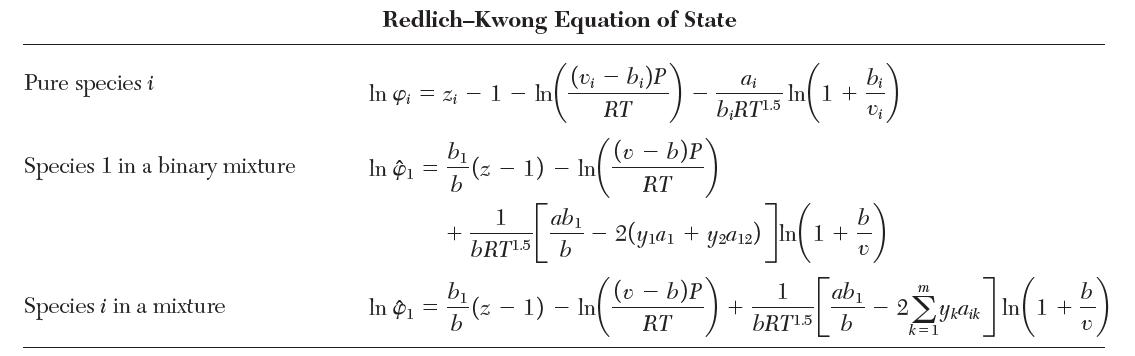
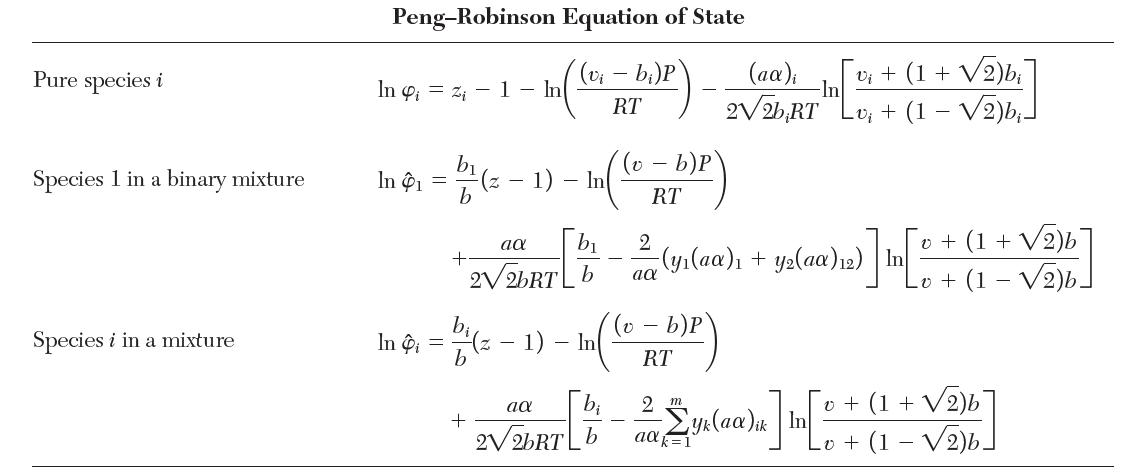
Consider a binary mixture of species 1 and 2 that obeys the Redlich–Kwong equation of state with van der Waals mixing rules. Show that the fugacity coeffi cient for species 1 is given by the “Species 1 in a binary mixture” row in Table 7.1.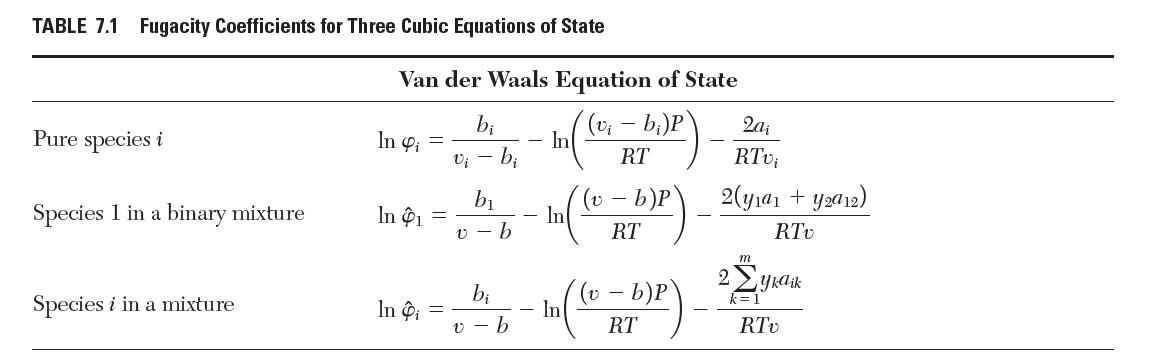
Transcribed Image Text:
TABLE 7.1 Fugacity Coefficients for Three Cubic Equations of State Pure species i Species 1 in a binary mixture Species i in a mixture Van der Waals Equation of State (v - b)P RT In 4 = In 1 In Pi = bi v - bi b b b v-b - - In - In In (v b)P RT v- b)P RT 2a RTvi 2(ya + y2a12) RTV 2 m yka ik RTv
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
An organ pipe is open at one end and closed at the other. The lowest note you can play on this pipe has frequency fmin. If the speed of sound in the pipe is v, what is the length L of the pipe? A L V...
-
Consider a binary mixture of species 1 and 2 that obeys the PengRobinson equation of state with van der Waals mixing rules. Show that the fugacity coeffi cient for species 1 is given by the Species 1...
-
Example 8.6 illustrates how you solve a dew-point calculation for a binary mixture of a nonideal liquid and a nonideal gas with T known. This problem corresponds to quadrant I in Figure 8.2. Develop...
-
AASB 102 prohibits the use of the LIFO method. What is the argument against the use of LIFO?
-
Read the Northwest Manufacturing Company Case in the Bodnar and Hopwood text. Using the information in this case: Prepare a flowchart of Northwest Manufacturing Company's current system for...
-
Axle Chemical Corporations treasurer has forecasted a $1 million cash deficit for the next quarter. However, there is only a 50 percent chance this deficit will actually occur. The treasurer...
-
Is there anything distinctive about the goals of public organizations? LO.1
-
Eminence Corporation makes rocking chairs. The chairs move through two departments during production. Lumber is cut into chair parts in the cutting department, which transfers the parts to the...
-
17. In a typical corporate organizational structure, which position is responsible for raising capital? . Which position is responsible for financial and managerial accounting and tax reporting?...
-
Calculate the fugacity and the fugacity coeffi cient of phenol in a mixture of 20 mole % phenol (1) and 80 mole % oxygen (2) at 694.2 K and 24.52 bar using the following: (a) The ideal gas law (b)...
-
Consider a ternary system of methane (a), ethane (b), and propane (c) at 25C and 15 bar. Assume this system can be represented by the virial equation truncated at the second term: At 25C, the second...
-
One of the variables in the experiment described in Problem 6-15, heat treatment method (C), is a categorical variable. Assume that the remaining factors are continuous. (a) Write two regression...
-
1. A concise introduction of the brand, including but not limited to a brief history, location information, size of the business, product/service offering, and so on.Give brief explanation. 2. Which...
-
A wood frame structure as shown to the right. The framingconsists of 2x6 studs, a single 2x6 bottom plate, two 2x6 topplates and a 2x10 joist. The studs are spaced at 16 in. on centerand sheathed...
-
In your reflection journal please list your order - 'most efficient' mediums at the top, 'least efficient' at the bottom. (for eg. social media, display ads, etc)Then, in five hundred words or more,...
-
Energy prices and global warming are discussed daily in the news as environmental impact of e-waste is just beginning to be recognized. Sustainability and corporate social responsibility need to be...
-
3. A Channel section is connected to a 10mm gusset plate with 20mm- diameter bolts as shown in the figure. The connecting member is subjected to dead load and live load only. The pitch distance,...
-
Describe the concept of customer lifetime value. Explain how consideration of this factor could be included in the GBE formula.
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
Estimate the temperature at which CuS045H,O undergoes dehydration.
-
For PbI2(s) = 0Pb+(aq) + 2 r(aq), K = 1.4 X 10-8 at 25C and the standard Gibbs energy of formation ofPbI2(s) is -173.64 k] mol ". Calculate the standard Gibbs energy of formation of PbI2 (aq).
-
Write the cell reaction and electrode half-reactions and calculate the standard emf of each the following cells: (a) Ptl C12 (g) I HCl (aq) 11 K, Cr04 (aq) IAg, Cr04(s) IAg (b) Pt 1 Fe3+(aq),Fe2+(aq)...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


