Consider a gas that undergoes a process from state 1 to state 2. You know the ideal
Question:
Consider a gas that undergoes a process from state 1 to state 2. You know the ideal gas heat capacity and an equation of state. Which of the following hypothetical paths would be most appropriate to chose to calculate Δu? Explain.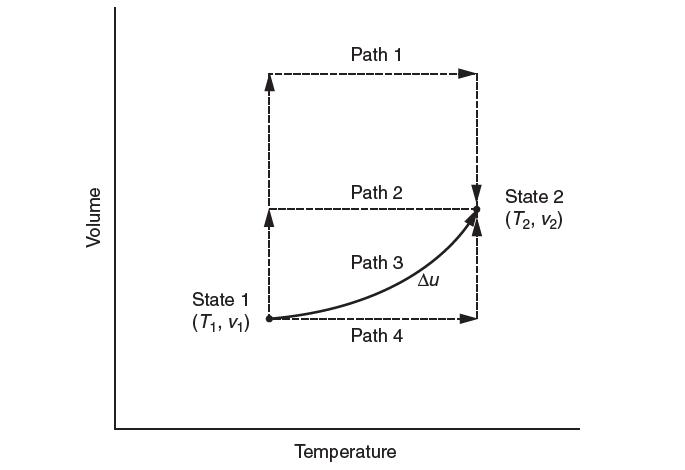
Transcribed Image Text:
Volume State 1 (T, V) Path 1 Path 2 Path 3 Path 4 Temperature Au State 2 (T2, V)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Yagyasini Sahu
I am a post graduate and having teaching experience of 6 years. Teaching is a passion for me and it gives a immense pleasure being with children and sharing my knowledge and experience with them. I have ability to motivate students.
I make students learning the concept very clearly in a smart way. because i believe that if anyone have fundamental knowledge, he/she can answer any type of question and will get good score. I always make them clear their doubts.The students who were poor in math have improved a lot after i taught them and got a good result, which makes me very happy.
I believe that knowledge and practice in a smartway is the keypoint to success.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider a gas that undergoes a process from state 1 to state 2. You know the ideal gas heat capacity and an equation of state. Which of the following hypothetical paths would be most appropriate to...
-
An ideal gas undergoes a process from state 1 to state 2. For the following data, approximate the change in specific entropy for this process using Eq. 7.13. State T(C) P (kPa) 1 200 2 180 100 150...
-
An ideal gas undergoes a process from state 1 to state 2. For the data shown below, approximate the change in specific entropy for this process using Eq. 7.11. (ft lbm) 4.5 3.2 State T(F) P (psia) 1...
-
Organizational buyers are ________.
-
Take a look at the US Census website, specifically https://www.census.gov/ and the chart labeled Quick Facts. Once you find your state, explore the information available. Choose one of these data...
-
Highland Company produces a lightweight backpack that is popular with college students. Standard variable costs relating to a single backpack are given below: Overhead is applied to production on the...
-
What two changing conditions led to the development of ERP systems? LO.1
-
Rockport Municipal Marina is considering adding a new dock to accommodate large yachts. The dock would cost $700,000 and would generate $144,000 annually in new cash inflows. Its expected life would...
-
Cutler Company has a cash account with a balance of $250,000 with Wright Bank and a cash account with an overdraft of $5,000 at Lowe Bank. What would the current assets section of Cutler's balance...
-
Consider the following property relation: (a) Come up with a physical process on a system which is described by the relation above. Sketch the process and describe it as completely as needed so that...
-
You are using the PengRobinson equation of state to determine the entropy change of an ideal gas: Is it better to try s = s (T,v) or s = s (T,P)? Explain. P = RT v-b aa(T) v(v + b) + b(v - b)
-
On which side of a trial balance would you find rent receivable?
-
According to a recent study, 21% of American college students graduate with no student loan debt. Suppose we obtain a random sample of 106 American college students and record whether or not they...
-
Differentiate the following with respect to x: a. y=5x+2x + x + 15 b. y=4x+3x - 4x - 10 c. y = 3Sin(5x) d. y = 3Cos(3x) e. y=10e -25x f. y = log(6x)
-
Question 2. The rate of drug destruction by the kidneys is proportional to the amount of the drug in the body. The constant of proportionality is denoted by K. At time t the quantity of the drug in...
-
5. 6. -1 (4a) U u X2 1 X2 -2 x -1 -2 12 (4b) U -2 2 Y y 16 x2 X2 3 1 (4c) U u - x 2 Y y -8 Y y -20 5 x X2 2 Find the state space models of the three systems shown in Fig. 4a, Fig. 4b, and Fig. 4c,...
-
Given the following data for Mehring Company, compute total manufacturing costs, prepare a cost of goods manufactured statement, and compute cost of goods sold. Direct materials used $230,000...
-
What is the concept of equity? How does it relate to consumer judgments of the fairness of a price?
-
The landing gear of an aircraft with: mass of 2000 kg the spring-mass-damper system Consider that the runway surface is y(t) = 0.2 cos 157.08t stiffness of the spring is 5 x 105 N/m. What is the...
-
Assess the validity of the following statement: the rate-determining step is the slowest step in a reaction mechanism.
-
Distinguish between kinetic and thermodynamic control of a reaction.
-
Distinguish between a primary and a secondary kinetic isotope effect. Discuss how kinetic isotope effects in general can provide insight into the mechanism of a reaction.
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


