You are using the PengRobinson equation of state to determine the entropy change of an ideal gas:
Question:
You are using the Peng–Robinson equation of state to determine the entropy change of an ideal gas: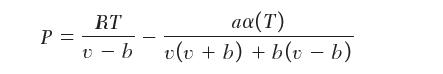
Is it better to try s = s (T,v) or s = s (T,P)? Explain.
Transcribed Image Text:
P = RT v-b aa(T) v(v + b) + b(v - b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Apple business is currently operating in the country of China and you were asked to report on different aspects of the business as you consider international expansion. By now, you should have a good...
-
On 01/01/2019 ABC Company purchase a truck for $140,000. The truck has a $50,000 salvage value and a life of 4 years with estimated total miles of 200,000. Fill in the answers below: 1. How much is...
-
A company had average total assets of $500,000, gross sales of $575,000, and net sales of $550,000. The companys total asset turnover is a. 1.15. b. 1.10. c. 0.91. d. 0.87. e. 1.05.
-
Choose three different presidents from Harry S. Truman through Barack Obama and look at the data. www.presidency.ucsb.edu/data/popularity.php?pres=44 For this, navigate to website's collection of job...
-
Developing Standard Costs ColdKing Company is a small producer of fruit-flavored frozen desserts. For many years, Cold- Kings products have had strong regional sales oa the basis of brand...
-
In the short run, how can capacity be changed? LO.1
-
A school district receives a grant from the federal government to support programs directed at special needs students. The grant is a matching grant in which each dollar spent by the school district...
-
John and Sally Claussen are considering the purchase of a hardware store from John Duggan. The Claussens anticipate that the store will generate cash flows of $70,000 per year for 20 years. John and...
-
Consider a gas that undergoes a process from state 1 to state 2. You know the ideal gas heat capacity and an equation of state. Which of the following hypothetical paths would be most appropriate to...
-
Of the following mixture, which do you think has entropy departure function of larger magnitude (a) 50 mol% methane mixed with 50 mol% ethane (b) 50 mol% acetone mixed with 50 mol% chloroform?...
-
Determine whether the series is convergent or divergent. 1 n + 4 n=1
-
Solve for "C" and "E": 1) E cos (15)-C=0 2) -300+ C+E sin (15) = 0
-
Let u=3, b. Compute uv, uv, 2-3 v =
-
Using Complex Numbers show that d cosz=-sinz dz
-
use for loops to solve the following problems 1. Write a complete C++ program that does the following. It asks the user to enter their age (which is assumed to be a positive integer). The program...
-
Profile Vickers hardness test Penetrating body: Square diamond pyramid :Test force F N ... 981 N (HV 5 ... HV 100) 49 :Measured value Diagonals of the square impression d Hardness value: F 0,189 F...
-
Describe some factors, in addition to size, that can affect the value of a perceived loss or gain.
-
How does the organizational structure of an MNC influence its strategy implementation?
-
The rate of the reaction A + 3 B --7 C + 2 D was reported as 1.0 mol dm-1 S-1. State the rates of formation and consumption of the participants.
-
The rate of consumption of B in the reaction A + 3 B 7 C + 2 D is 1.0 mol dm3 S-1. State the reaction rate, and the rates of formation or consumption of A, C, and D.
-
The rate law for the reaction in Exercise 22.1b was found to be v = k[A][BF, What are the units of k? Express the rate law in terms of the rates of formation and consumption of (a) A, (b) C.
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


