Consider a gas that undergoes a process from state 1 to state 2. You know the ideal
Question:
Consider a gas that undergoes a process from state 1 to state 2. You know the ideal gas heat capacity and an equation of state. Which of the following hypothetical paths would be most appropriate to chose to calculate Δh? Explain.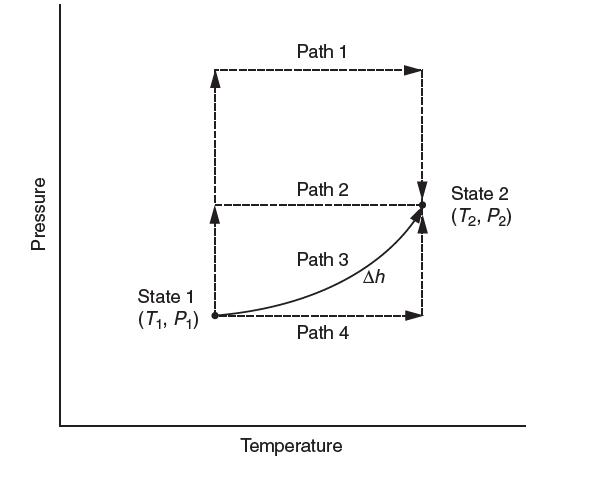
Transcribed Image Text:
Pressure State 1 (T, P) Path 1 Path 2 Path 3 Path 4 Temperature Ah State 2 (T2, P2)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Rayan Gilbert
I have been teaching since I started my graduation 3 years ago. As a student, working as Teacher/PA has been tough but made me learn the needs for student and how to help them resolve their problems efficiently. I feel good to be able to help out students because I'm passionate about teaching. My motto for teaching is to convey the knowledge I have to students in a way that makes them understand it without breaking a sweat.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider a gas that undergoes a process from state 1 to state 2. You know the ideal gas heat capacity and an equation of state. Which of the following hypothetical paths would be most appropriate to...
-
An ideal gas undergoes a process from state 1 to state 2. For the following data, approximate the change in specific entropy for this process using Eq. 7.13. State T(C) P (kPa) 1 200 2 180 100 150...
-
An ideal gas undergoes a process from state 1 to state 2. For the data shown below, approximate the change in specific entropy for this process using Eq. 7.11. (ft lbm) 4.5 3.2 State T(F) P (psia) 1...
-
The United StatesMexicoCanada Agreement replaced what trade agreement?
-
LMX theory assumes that improved exchanges between leaders and followers are desirable. When might a follower not want "improved career-oriented social exchanges" with a leader? Describe one...
-
Fleming, chief administrator for Valley View Hospital, is concerned about the costs for tests in the hospitals lab. Charges for lab tests are consistently higher at Valley View than at other...
-
What is MRP II? LO.1
-
A successful California engineer has installed a circular hot tub in his back yard and finds that for the typical operating conditions prescribed below, water must be added at a rate of 0.00 I kg/s...
-
The following information was taken from the accounts of Green Market, a delicatessen, at December 31, Year 2. The accounts are listed in alphabetical order, and each has a normal balance. Req A Req...
-
Consider the following property relation: (a) Come up with a physical process on a system which is described by the relation above. Sketch the process and describe it as completely as needed so that...
-
You are using the PengRobinson equation of state to determine the entropy change of an ideal gas: Is it better to try s = s (T,v) or s = s (T,P)? Explain. P = RT v-b aa(T) v(v + b) + b(v - b)
-
Why is a public announcement of numerical inflation rate objectives important to the success of an inflation-targeting central bank?
-
Can anyone explain me how to calculate the ROI using the HISTORICAL COST NBV, the formula my instructor wants me to use is ADJ CF - HIST DEP /ASSETTOTAL - ACC DEP. And for the ROI of CURRENT COST NBV...
-
Consider the circuit to the right 3. If the total voltage supply in the circuit is 120V, and each resistor has a resistance of 400, what will the current read on each ammeter? |1= 12= 3 = 4. What...
-
1. The theory predicts the proportion of beans, in the four groups A, B, C and D should be 9:3:3:1. In an experiment among 1600 beans, the numbers in the four groups were 882, 313, 287 and 118. Does...
-
Would you recommend criminal charges in this case ( the screenshots below) and, if so, exactly which statutes against which person? Explain your reasoning (how the elements of the crime are met or...
-
check if each transaction is placed in the right place in each of the reports below and if there are any other mistakes in the different accounts after the first image which is a description of the...
-
Describe some factors, other than the consumers impression of the sellers profits, that contribute to judgments of price fairness.
-
XYZ Inc. a calendar year, accrual basis corporation, had the following items during 2021: Gross revenue from operations Cost of goods sold $420,000 ($180,000) $9,000 LT capital gain .LT capital...
-
In this problem, we examine a model for the transport of oxygen from air in the lungs to blood. First, show that, for the initial and boundary conditions c(x, t) = c(x, 0) = co (0 < x < ] and c(0, t)...
-
Consult literature sources and list the observed timescales during which the following processes occur radiative decay of excited electronic states, molecular rotational motion, molecular vibrational...
-
Describe the main features, including advantages and disadvantages, of the following experimental methods for determining the rate law of a reaction: the isolation method, the method of initial...
-
Nitin is paid a base salary of $200 per week and commission at the rate of 3% for sales over $5000, 4% if his sales are over $8000, and 5% if sales are over $15,000. How much will Nitin earn in a...
-
Safa is paid a base salary of $1500 per month and a commission of 6% on all sales over $75,000. Last month, Safa's gross salary was $4440. What were her sales for the month? a$149,000 b$124,000...
-
Your regular hourly rate of pay is $15.86, and you are paid double time for all work on weekends and for any time over forty hours per week (Monday to Friday). Calculate your gross earnings for a...

Study smarter with the SolutionInn App


