Consider an ideal, reversible magnetic refrigeration cycle shown in the fi gure on next page. A paramagnetic
Question:
Consider an ideal, reversible magnetic refrigeration cycle shown in the fi gure on next page.
A paramagnetic working material in the form of the rim of a wheel is rotated between a hightemperature reservoir and a low-temperature reservoir. In the high-temperature reservoir, the working material expels heat into the reservoir as it is subjected to a high magnetic fi eld. As the working material moves into the low-temperature reservoir, the fi eld becomes smaller and eventually zero.
The demagnetization process causes the working fl uid to absorb heat from the low-temperature reservoir. The working material is gadolinium sulphate octahydrate. On the upper-left-hand side of the fi gure, helium enters the porous wheel at temperature 1.1 K and a magnetic fi eld of 0.9 tesla [T] and is forced to fl ow in heat exchange with the moving wheel. The wheel absorbs heat from the helium as it is demagnetized. The temperature of the helium drops 0.2 K during this process. The helium then absorbs heat QC from the load and reenters the heat exchanger area at 1.1 K. Similarly, in the lower-right-hand side of the fi gure, helium enters the porous wheel at 8 K and a magnetic fi eld of 1.6 T. The wheel deposits heat into helium as the material is magnetized. The temperature rises to 9.5 K at 6.4 T.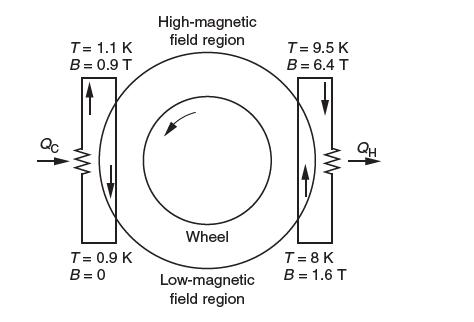
Approximately how much heat is being expelled by the cold reservoir? How much heat is being absorbed by the hot reservoir? Estimate the coeffi cient of performance for this process.
How does the numerical value compare with a conventional refrigeration cycle? How is work being supplied to perform the refrigeration process? A Ts diagram for gadolinum sulphate octahydrate is provided.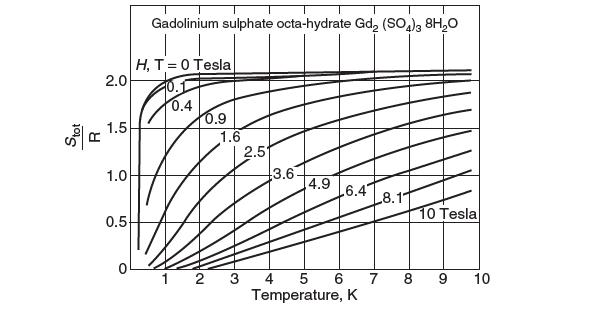
Step by Step Answer:






