Consider the oxidation of cuprous oxide to form cupric oxide by the following reaction: Calculate srxn. This
Question:
Consider the oxidation of cuprous oxide to form cupric oxide by the following reaction:![]()
Calculate Δsrxn. This task can be done in the same type of path described in Section 2.6 for Δhrxn.
You can calculate the values of entropies of formation from the data in Appendix A.3 by applying the following relationship: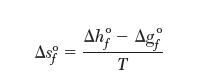
Physically explain the sign of Δsrxn. Does the formation of CuO violate the second law of thermodynamics? Explain.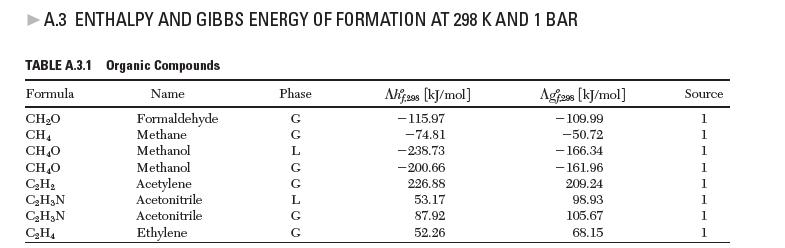
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





