Consider the use of CF2Cl2 as a dispersing agent for aerosol spray cans. Estimate the pressure a
Question:
Consider the use of CF2Cl2 as a dispersing agent for aerosol spray cans. Estimate the pressure a can has to withhold at 40°C. Its enthalpy of vaporization at its normal boiling point (244 K) is 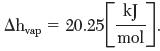
State your assumptions.
Transcribed Image Text:
Ahvap 20.25 kJ mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The Company XYZ has 1173 blocks of building for its business operation, where each block has 7 floors. The distance between each floor is 7 meters. ] (ii) Give a function run2diff which can be...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
The Compiler Construction Programming answers should be written in some notation approximating SML or OCaml. (a) Describe what is meant by tail recursion. [4 marks] (b) Eliminate tail recursion from...
-
For each of the following tests, identify two different samples of people who would have the expertise to serve as subject matter experts (SMEs) for providing judgments regarding the content validity...
-
Write a paper on "Should regulations regarding the use of cell phones be standardized"
-
Gold Nest Company of Guandong, China, is a family-owned enterprise that makes birdcages for the South China market. A popular pastime among older Chinese men is to take their pet birds on daily...
-
Describe the Project Management Body of Knowledge (PMBOK) and be familiar with its knowledge areas and process groups. AppendixLO1
-
The data shown in Table 6E.4 are the deviations from nominal diameter for holes drilled din a carbon-fiber composite material used in aerospace manufacturing. The values reported are deviations from...
-
Whirly Corporation's contribution format income statement for the most recent month is shown below: 30 points Sales (8,700 units) Variable expenses Contribution margin Fixed expenses Net operating...
-
At 1 atm titanium melts at 1941 K and boils at 3560 K. Its triple point pressure is 5.3 Pa. Using only these data, estimate the enthalpy of vaporization of titanium. You will need to think about a...
-
A TP diagram of carbon is presented in the following fi gure. The following data are available at 25C. Answer the following questions: (a) Identify the region where diamond is the thermodynamically...
-
Discuss the purpose of the standard coding guidelines.
-
9. [10] Suppose that B and W are BMs and that they are correlated with correlation coefficient P (-1, 1) in the sense that the correlation coefficient between Bt and Wt for all t>0. Then we can...
-
You have just incorporated and started your business. Your corporate pre-tax profit is $40,000. This is your only source of income. This income is eligible for the Small Business Deduction and is...
-
4. Provide the information requested in the statements below: a) Find and draw all C's that do not contain H's (if any). For this, redraw the structure where you show the d ('s). N b) Find and draw...
-
Suppose that f(x) = 8x + 5. (A) Find the slope of the line tangent to f(x) at x = 7. (B) Find the instantaneous rate of change of f(x) at x = -7. C) Find the equation of the line tangent to f(x) at x...
-
Whichof the following regarding the relationship between business risk and financial risk is least accurate based on our discussions in class? A. Business risk represents uncertainty caused by...
-
How might factors such as customer income and competitive intensity be used in defining retail price zones?
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Ozone absorbs ultraviolet radiation in a part of the electromagnetic spectrum energetic enough to disrupt DNA in biological organisms and that is absorbed by no other abundant atmospheric...
-
G.C.G. Wachewsky, R. Horansky, and V. Vaida (J. Phys. Chem. 100, 11559 (1996)) examined the UV absorption spectrum ofCH3I, a species of interest in connection with stratospheric ozone chemistry. They...
-
One of the principal methods for obtaining the electronic spectra of unstable radicals is to study the spectra of comets, which are almost entirely due to radicals. Many radical spectra have been...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


