Develop a general expression for ssys for an ideal gas that goes from (v1, T1) to (v2,
Question:
Develop a general expression for Δssys for an ideal gas that goes from (v1, T1) to (v2, T2) based on the path below.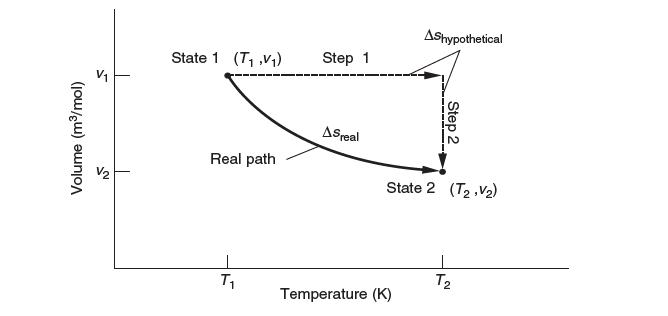
Transcribed Image Text:
S Volume (m/mol) NT State 1 (T.V) Real path T Step 1 ASreal AShypothetical Temperature (K) Step 2 State 2 (T, V) T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
An ideal gas flows adiabatically through a duct. At section 1, p1 = 140 kPa, T1 = 260°C, and V1 = 75 m/s. Farther downstream, p2 = 30 kPa and T2 = 207°C. Calculate V2 in m/s and s2 − s1...
-
A gas has an ideal gas heat capacity of C P * = (7/2)R and is described by the equation of state: Z = 1 + (CP 2 )/(RT) with C = 100 cm 3 /bar mol. A . Find a general expression for the residual...
-
The graph of f is given. (a) Why is f one-to-one? (b) What are the domain and range of f 1 ? (c) What is the value of f 1 (2)? (d) Estimate the value of f -1 (0). 1
-
Yates Company has a noncontributory, defined benefit pension plan. It is December 31, 1998, end of the accounting year and measurement date for the pension plan. The following are the data for three...
-
Text categorization is the task of assigning a given document to one of a fixed set of categories, on the basis of the text it contains. Naive Bayes models are often used for this task in these...
-
Why do we need evaluation measures for cluster algorithms?
-
Early in September 1983, it took 245 Japanese yen to equal $1. Nearly 25 years later, in May 2008, that exchange rate had fallen to 103.5 yen to $1. Assume that the price of a Japanese-manufactured...
-
Inventory Analysis The following data were extracted from the income statement of Keever Inc. Current Year Previous Year Sales $1,430,800 $1,498,000 Beginning inventories 63,564 75,708 Cost of goods...
-
Develop a general expression for Dssys for an ideal gas that goes from (P1, T1) to (P2, T2) where heat capacity is given by: Cp = A + BT + CT2
-
The concept of entropy was developed in the nineteenth century, in order to study the efficiency of the steam engine, largely through the work of Sadi Carnot, Rudolph Clausius, and Lord Kelvin....
-
The tension T in the string of the yo-yo in the figure is T = mtR/2r 2 + R 2 where m is the mass of the yo-yo and g is acceleration due to gravity. Use differentials to estimate the change in the...
-
7. This is a question about electromagnetic waves. (a) Starting from Maxwell's equations in a vacuum show that the electric field E and magnetic field B obey wave equations and identify the velocity...
-
Participate in workplace health and safety Third- party report Task 1: Case scenario: Workplace hazard collection and risk control form You are required to review this workplace inspection form and...
-
The red curve is the position-time x-t graph for the ladybug. Each tick mark on the time axis of the graph marks off 0.5 s. Note: you can hit reset graph and graph again to watch the graph form again...
-
Oma's Bakery is thinking about replacing the convection oven with a new, more energy-efficient model. Information related to the old and new ovens follows: (Click the icon to view the information...
-
One could argue that substantial travel for work is an undesirable characteristic of any job. What would the theory of compensating differentials predict about the relative wages of a sales position...
-
In a recent study, when asked to choose between an iPod and $100, people were more likely to choose the money. But when they were given an iPod and then asked if they would trade it for $100, they...
-
Cassandra Casey operates the Futuristic Antique Store. She maintains subsidiary ledgers for accounts payable and accounts receivable. She presents you with the following information for October 2019:...
-
When D-galactose was heated at 165C, a small amount of compound A was isolated:
-
Phlorizin is obtained from the root bark of apple, pear, cherry, and plum trees. It has the molecular formula C21H24O10 and yields a compound A and D-glucose on hydrolysis in the presence of emulsin....
-
Emil Fischers determination of the structure of glucose was carried out as the nineteenth century ended and the twentieth began. The structure of no other sugar was known at that time, and none of...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


