The following plot shows values of excess Gibbs energy over the ideal gas constant, gE/R in [K],
Question:
The following plot shows values of excess Gibbs energy over the ideal gas constant, gE/R in [K], for mixtures of benzene–cyclohexane at 343 K. Answer the following questions:
(a) Estimate the activity coeffi cient of cyclohexane in benzene (1)–cyclohexane (2) at 343 K for
(i) a mole fraction of cyclohexane of x2 = 0.25; (ii) cyclohexane in infi nite dilution.
(b) Estimate the Henry’s law constant for cyclohexane in benzene.
(c) Are the like interactions stronger or weaker than the unlike interactions? Explain.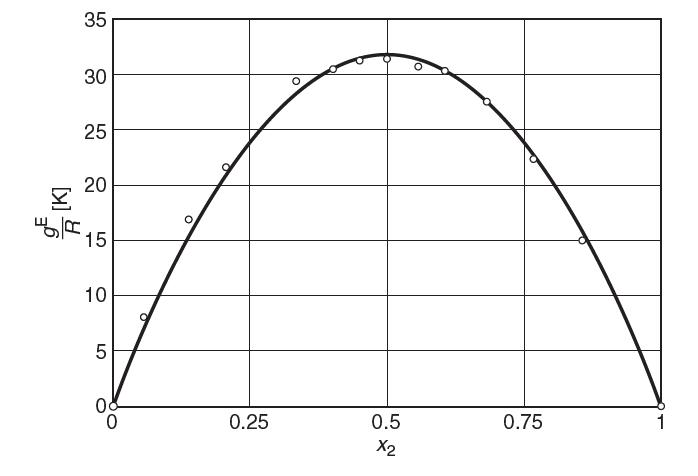
Step by Step Answer:
Related Book For 

Question Posted:




