The partial molar volumes of a binary mixture of ethanol (1) and ethylene glycol (2) at 25C
Question:
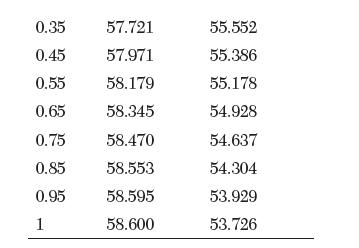
The partial molar volumes of a binary mixture of ethanol (1) and ethylene glycol (2) at 25°C are reported in the following table. Answer the following questions: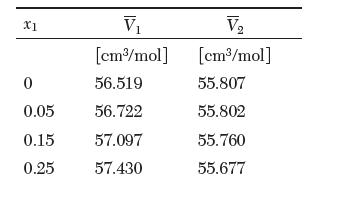

Transcribed Image Text:
V [cm3/mol] 0 56.519 0.05 56.722 0.15 57.097 0.25 57.430 X1 V [cm/mol] 55.807 55.802 55.760 55.677
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
As an undergraduate chemical engineering student, you are involved in a summer research project that requires you to measure the molar volume of a methanol (1) + water (2) binary system at 298.15 K...
-
The molar volume, in [cm3/mol], of a binary mixture of ethanol (1) and ethylene glycol (2) at 25C is given in the following table. Using the graphical method, determine the partial molar volume of...
-
Using the information in Problems 7.13 and 8.20, estimate the heat of vaporization for the first bit of ethanol from ethanol-water solutions containing 25, 50, and 75 mol % ethanol and from a...
-
Based on Exhibit 1, the expected future value of Bond I at maturity is closest to: A. 98.80. B. 103.74. C. 105.00. Lena Liecken is a senior bond analyst at Taurus Investment Management. Kristel...
-
Compare the drug abuse climate in the United States before and after the passage of Prohibition. Video link https://www.youtube.com/watch?v=rIWwHjm-F_o
-
Balanced scorecard Following is a random-order listing of perspectives, strategic objectives, and performance measures for the balanced scorecard. For each perspective, select those strategic...
-
EXERCISE 21 Compute a Predetermined Overhead Rate LO21 Harris Fabrics computes its plantwide predetermined overhead rate annually on the basis of direct labor-hours. At the beginning of the year, it...
-
Hiring an employee and taking a sales order are business activities but are not accounting transactions requiring journal entries. Make a list of some other business activities that would not be...
-
of 4 Required information [The following information applies to the questions displayed below.) North Inc. is a calendar-year C corporation, accrual-basis taxpayer. At the end of year 1, North...
-
Enthalpies of solution, are reported in Table 6.1 for 1 mole of HCl diluted in n moles of H2O at 25C: (a) Consider a mixture of 8 moles H2O and 2 moles HCl. As best you can from these data, estimate...
-
A binary mixture of species a and b behaves as an ideal gas at 300 K and 1 bar. Calculate the partial molar Gibbs energy of species a, Ga, and the total solution Gibbs energy, g, at the following...
-
The body mass index is defined in Exercise 39. Draw the level curve of this function corresponding to someone who is 200 cm tall and weighs 80 kg. Find the weights and heights of two other people...
-
Question (4) seen, 20 vehicles/km moving at 100 km/h and 30 vehicles/km traveling at 120 km/h. Two successive videos showing stationary traffic on the road were examined. Two groups of platoons were...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Explain the process of compression resin transfer molding(CRTM)?in composite manufacturing. What are the benefits of using CRTM for producing composite structures?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
Describe what is true when two products interact as complements.
-
F.(3e* -2x 3 sin(2x)) is equal to 2 3 Cos 8. IT 3, t (4+@ 2 3, 1+o 1 4 Cos 4 4 1 3. 1 +4cos V7 (1+o 4 1 4 Cos 4 1+0 4-
-
The algebraic forms of the [orbitals are a radial function multiplied by one of the factors (a) z (5z2 - 3r2), (b) y (5y2 - 3r2), (c) x (5x2 - 3r2), (d) Z(x2 Y2), (e) Y(x2- z2), (f) x (z2 y2), (g)...
-
The NO2, molecule belongs to the group C2v' with the C2 axis bisecting the ONO angle. Taking as a basis the N2s, N2p, and 02p orbitals, identify the irreducible representations they span, and...
-
The phenanthrene molecule (29) belongs to the group C2v with the C, axis in the plane of the molecule. (a) Classify the irreducible representations spanned by the carbon 2pz orbitals and find their...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


