You need to fi nd the enthalpy of sublimation of solid A at 300 K. The following
Question:
You need to fi nd the enthalpy of sublimation of solid A at 300 K. The following equilibrium vapor pressure measurements have been made on pure A: (1) at 250 K, the pressure is 0.258 bar and (2) at 350 K, the pressure is 2.00 bar. The following heat capacity data are known: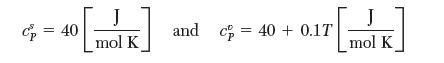
(a) Calculate the enthalpy of sublimation, assuming Δhsub is constant.
(b) Calculate the enthalpy of sublimation, accounting for the temperature variation of Dhsub.
(c) Estimate the error in the constant T assumption.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





