A hexagonal molecule has angles of 120 inside the ring and is flat. The following two molecules
Question:
A hexagonal molecule has angles of 120° inside the ring and is flat. The following two molecules are often drawn as flat hexagons, but only one is truly flat. Which one is it and why?
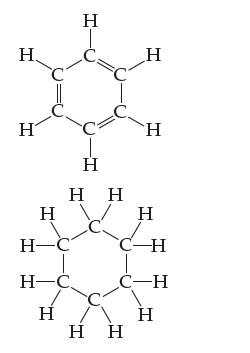
Transcribed Image Text:
Η. H Η H-C Η Η H¬C Η Ι Η Η Η H Ἡ Η C-H Η C-H Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The molecule on the left is truly flat because it has angles of 120 inside the ringwhich is consiste...View the full answer

Answered By

Ashish Bhalla
I have 12 years work experience as Professor for Accounting, Finance and Business related subjects also working as Online Tutor from last 8 years with highly decentralized organizations. I had obtained a B.Com, M.Com, MBA (Finance & Marketing). My research interest areas are Banking Problem & Investment Management. I am highly articulate and effective communicator with excellent team-building and interpersonal skills; work well with individuals at all levels.
4.80+
17+ Reviews
46+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
A uniformly charged thin ring has radius 15.0 cm and total charge +24.0 nC. An electron is placed on the ring's axis a distance 30.0 cm from the center of the ring and is constrained to stay on the...
-
A container of weight W is suspended from ring A. Cable BAC passes through the ring and is attached to fixed supports at B and C. Two forces P= Pi and Q= Qk are applied to the ring to maintain the...
-
Find the derivative of the vector function r(t) = e^t^2 i-j + In(1+3t)k.
-
Go to the text Web site at www.cengage.com/accounting/vanderbeck and click on the link to kaizen, from Wikipedia, the free encyclopedia. After reading the entry, answer the following questions: 1....
-
Health 'R Us, Inc., uses a traditional product costing system to assign overhead costs uniformly to all its packaged multigrain products. To meet Food and Drug Administration requirements and to...
-
Is the contractor licensed and insured?
-
The following data relate to the operations of Soper Company, a wholesale distributor of consumer goods, as of March 31: Cash. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $...
-
*UPDATED* This is all the information I have Vaughn Industries had sales in 2021 of $5,739,200 and gross profit of $928,400. Management is considering two alternative budget plans to increase its...
-
Consider CH 3 + and CH 3 . Using these as examples, explain why it is necessary to draw a correct Lewis dot diagram before trying to predict the shape of a molecule. Use the concept of steric number...
-
Consider the following molecule. The diagram shows how the atoms are connected, but it is not a complete dot diagram. (a) Complete the dot diagram. (b) Redraw the molecule showing its three...
-
What are 'international assignments' and why do multinational corporations use them?
-
Factor completely. x10 2x5 +1
-
Which of the three essential financial statements is most important for your business for the feasibility plan? Explain your answer.
-
Module 05 Content Background Having a comprehensive understanding of curriculum models and approaches to early childhood education can give you an appreciation of the many options available to...
-
- 7 ( 3 x - 2 ) 2 find the derivative
-
The Reciprocal Method Solve the Simultaneous Equations: S1=170,000+(0.2*S2) S2=68,000+(0.2*S1) S1=170,000+[0.2*(68,000+0.2*S1)]
-
Show that for a plane curve N points to the concave side of the curve. One method is to show that Then consider the cases dÏ/ds > 0 (curve bends to the left) and dÏ/ds doids doids
-
Why is inventory management important for merchandising and manufacturing firms and what are the main tradeoffs for firms in managing their inventory?
-
How would you use IR spectroscopy to distinguish between the following pairs of compounds? (a) (b) N.
-
The amplitude of a standing wave function representing a moving particle can change from positive to negative values in the domain (0, a) over which the wave function is defined. It must therefore...
-
According to the 3rd postulate, in any single measurement of the total energy, the only values that will ever be measured are the eigenvalues of the total energy operator. Apart from the discrete...
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


