A zinc electrode and a copper electrode are used to make a battery, as shown below: (a)
Question:
A zinc electrode and a copper electrode are used to make a battery, as shown below:
(a) Replace the three question marks with appropriate labels.
(b) Indicate which way the electrons flow.
(c) Write the spontaneous redox reaction.
(d) Add the labels +, –, anode, and cathode to the drawing.
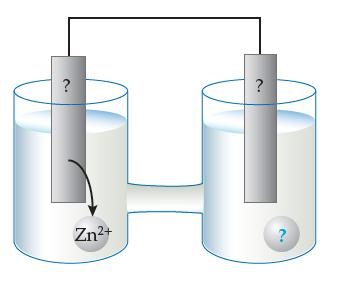
(e) Write “oxidation occurs at this electrode”
under the appropriate beaker.
(f) Write “reduction occurs at this electrode”
under the appropriate beaker.
(g) Indicate in which beaker the concentration of metal ions increases with time.
(h) Indicate in which beaker the concentration of metal ions decreases with time.
(i) Indicate in which beaker the electrode appears eaten away with time.
(j) Indicate in which beaker the electrode gains mass with time.
Step by Step Answer:

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver





