Balance each equation and classify the reaction as combination, decomposition, single replacement, or double-replacement. H H C=C
Question:
Balance each equation and classify the reaction as combination, decomposition, single replacement, or double-replacement.
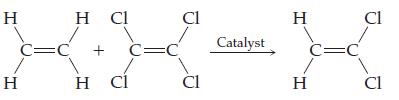
Transcribed Image Text:
H H C=C H Cl + H Cl C=C Cl CI Catalyst H H C=C Cl Cl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The IPRC-DFRD-funded pilot project is not going as well as planned. The project manager is quite unsure about the outcome of any present phases. Team members seem to be frustrated about the project...
-
WHAT IS THE CORRECT ANSWER? A) 5.21 B) -15 C) 57.6 D) -62.1 E) 0.37 Refrigerant 134a flows through a horizontal pipe. Inlet: T = 40C P = 280 kPa 1 Outlet: T = 40C P = 240 kPa The mass flow rate is...
-
Balance each equation and classify the reaction as combination, decomposition, single replacement, or double-replacement. KBr(aq) + Cl 2 (aq) KCl(aq) + Br 2 (aq)
-
Use the Ratio Test to determine the values of x 0 for which each series converges. 00 X 2k 2 k=1 k
-
Estimating fixed and variable costs using the high-low method Conry Ice Cream Company produces various ice cream products for which demand is highly seasonal. The company sells more ice cream in...
-
1. What is the problem facing Purinex, Inc.s CFO Gilad Harpaz? What is the urgency? 2. What is Purinexs business, and how would you describe its strategy? What do you think are its founders goals and...
-
Major depression and personality disorders. A team of physicians, psychiatrists, and psychologists investigated whether depressed patients exhibit more or fewer personality disorder symptoms than...
-
In a pure exchange economy with two goods, G and H, the two traders have Cobb-Douglas utility functions. Suppose that Tony's utility function is Ut = GtHt and Margaret's utility function is Um =...
-
The following are selected current month's balances for Allbright Enterprises. Accounts Payable Revenue Cash Expenses Furniture Accounts Receivable Common Stock Notes Payable $7,000 10,000 3,000...
-
To write a net ionic equation, you must (a) Write every species in solution. (b) Include the spectator ions. (c) Omit the spectator ions. (d) Write the precipitate with (aq) after it.
-
Balance each equation and classify the reaction as combination, decomposition, single replacement, or double-replacement. Pb(NO3)2(s) Heat PbO(s)+NO(g)+O(g)
-
On December 31, 2016, BIG Company had accrued salaries of $6,400. Required a. Record in general journal format the adjustment required as of December 31, 2016. b. Record the above adjustment in a...
-
Nequired information Exercise 5-17 (Static) Notes receivable-interest accrual and collection LO 5-6 (The following information applies to the questions displayed below) Agrico Incorporated accepted...
-
Case 14-3 Sarin Pharmaceuticals Ltd. Alan Mannik, director of procurement for the Sarin Phar- maceuticals Ltd. (Sarin) Animal Health Division plant in Vancouver, British Columbia, was planning for...
-
CL727 LEGAL ANALYSIS AND WRITING Module 11 Assignment: Brief Answer, Analysis, and Conclusion This assignment will be due in Module 11. Your assignment is to write the Brief Answer, Analysis, and...
-
Question 11 (0.5 points) l) Listen } As a drug manufacturer, you expect your latest wonder drug to lower cholesterol. It has been successful with a limited group of participants so far, so you have...
-
Redfern Audio produces audio equipment including headphones. At the Campus Facility, it produces two wireless models, Standard and Enhanced, which differ both in the materials and components used and...
-
Under what circumstances can you assume that the less stable reactant will be the more reactive reactant?
-
Per Bag Direct materials: 25 pounds of CWhiz-2000 @ $0.08/lb. = $ 2.00 Direct labor: 0.05 hour @ $32.00/hr. = $ 1.60 The company manufactured 100,000 bags of Cheese-Be-Good in December and used...
-
(a) Draw a block diagram for a floating-point subtracter. Assume that the inputs to the subtracter are properly normalized, and the answer should be properly normalized. The fractions are 8 bits...
-
a) Perform the floating point addition. b) Draw an SM chart for a floating-point adder that adds F 1 x 2 E1 and F 2 x 2 E2 . Assume that the fractions are initially normalized (or zero) and the final...
-
For the floating-point adder of Figure 7-14, modify the Verilog code so that (a) It handles IEEE standard single precision denormalized numbers both as input and output. (b) In state 2, it speeds up...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


