Circle the correct choice to indicate how many electrons each element must gain or lose to form
Question:
Circle the correct choice to indicate how many electrons each element must gain or lose to form an octet:
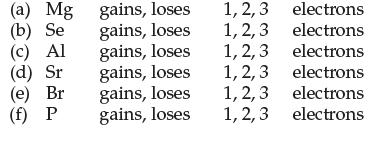
Transcribed Image Text:
(a) Mg (b) Se (c) Al (d) Sr (e) Br (f) P gains, loses gains, loses gains, loses gains, loses gains, loses gains, loses 1, 2, 3 1, 2, 3 1, 2, 3 1, 2, 3 1, 2, 3 1, 2, 3 electrons electrons electrons electrons electrons electrons
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Answer a Mg lose 2 electrone to from an octet because The electronic configuration of Mg is 1s 2 2s ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
1 points Question 11 Your 91-year-old great-aunt has terminal cancer. You and shehave had many conversations regarding what she would want done ifshe were incapacitated and could not make choices...
-
Marblehead Manufacturing, Inc., has two departments, Mixing and Blending. When goods are completed in Mixing, they are transferred to Blending and then to the finished goods storeroom. There was no...
-
What do the numbers on financial statements actually represent?
-
Palmer Corporation, operating as a U.S. corporation, expects to order goods from a foreign supplier at a price of 200,000 pounds, with delivery and payment to be made on April 15. On January 15,...
-
At December 31, 2013, Obermeyer Imports reported the following information on its balance sheet. Accounts receivable $250,000 Less: Allowance for doubtful accounts 15,000 During 2014, the company had...
-
Instructions X The following selected transactions were completed by Amsterdam Supply Co., which sells office supplies primarily to wholesalers and occasionally to retail customers. Also note that...
-
What are the group number, period number, and name of the element whose electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 2 ?
-
If gamma radiation has a wavelength of 1.00 10 12 m, what is the energy of gamma radiation in joules?
-
The BlackScholesMerton price of an out-of-the-money call option with an exercise price of $40 is $4. A trader who has written the option plans to use a stop-loss strategy. The traders plan is to buy...
-
1. What are the threats being faced by Indian General Insurance Ltd. (IGIL)? 2. What are its traditional strengths? What 'business definitions' should it follow while capitalizing on its traditional...
-
You go to discuss the incident and the client's claims with your supervisor. As you retell the incident, it is clear that your supervisor is not comfortable. You ask your supervisor for advice on the...
-
Case Study Two: Rawlings Rawlings is an American sports equipment manufacturing company based in Town and Country, Missouri, and founded in 1887. Rawings specializes in baseball equipment and...
-
The discussion is for Administrating organizational change course. (we should write 300 words) Discussion question is: Refer to table 6.4 in your book. Think of a time when you were introduced to...
-
Content: Identify at least two resources for each of the four critical sections in the course project: Strategic Planning, Healthcare Reimbursement, Revenue Cycle Process, and Reimbursement...
-
If a = (3, 3, 1), b = (-2, -1, 0), and c = (-2, -3, -1), find each of the following: a. a b b. a (b +c) c. a (b c) d. a (b c)
-
TRUE OR FALSE: 1. Banks with a significantly large share of fixed-interest rate home loans are less exposed to interest rate risks. 2. Although Australian banks are pretty big, they are not...
-
In Figure 19.16, n J /n 0 increases initially with J for all three temperatures for CO, but only for the two highest temperatures for HD. Explain this difference. Figure 19.16 HD CO 2.5 12 10 2.0...
-
What is the difference between the transition dipole moment and the dynamic dipole moment?
-
Nitrogen and oxygen do not absorb infrared radiation and are therefore not greenhouse gases. Why is this the case?
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


