Complete the table: Solute Solute mass (g) NaBr 3.96 Ba(OH)2 2.58 (NH4)2SO4 8.65 NH4Cl Solution Molarity volume
Question:
Complete the table:
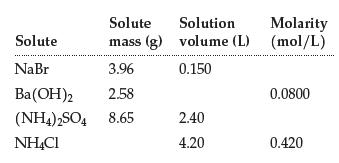
Transcribed Image Text:
Solute Solute mass (g) NaBr 3.96 Ba(OH)2 2.58 (NH4)2SO4 8.65 NH4Cl Solution Molarity volume (L) (mol/L) 0.150 2.40 4.20 0.0800 0.420
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Molarity M mass g 1 molar mass gmol Liter Molarity M NaBr 396 ...View the full answer

Answered By

Irfan Ali
I have a first class Accounting and Finance degree from a top university in the World. With 5+ years experience which spans mainly from the not for profit sector, I also have vast experience in preparing a full set of accounts for start-ups and small and medium-sized businesses. My name is Irfan Ali and I am seeking a wide range of opportunities ranging from bookkeeping, tax planning, business analysis, Content Writing, Statistic, Research Writing, financial accounting, and reporting.
4.70+
249+ Reviews
530+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
For an ideal gas, the decrease in internal energy of 1.4 kgm is -343KJ when the volume increases from 0.043 cu. m. to 0.13 from 0.07 bar to 0.02 bar; c, = 0.72 cu. m. and the pressure decreases kgm-K...
-
Complete the table below for contribution margin per unit, total contribution margin , and contribution margin ratio: A Number of units 1,720 units 14,920 units 4,620 units Sales price per unit $...
-
Problem 13-1A Calculation and analysis of trend percents LO A1, P1 Selected comparative financial statements of Haroun Company follow. HAROUN COMPANY Comparative Income Statements For Years Ended...
-
Why will a reduction in the real interest rate increase investment spending, other things equal?
-
A machine is purchased January 1 at a cost of $58,000. It is expected to produce 110,000 units and have a salvage value of $3,000 at the end of its useful life. Units produced are as follows: Year 1...
-
What are the differences between GAAP based and Pro Forma financial statements?
-
College freshmen. A survey of college freshmen in 2001 asked what field they planned to study. The results: 12.6%, arts and humanities; 16.6%, business; 10.1%, education; 18.6%, engineering and...
-
PowerTrain Sports Inc. manufactures and sells two styles of All Terrain Vehicles (ATVs), the Mountain Monster, and Desert Dragon from a single manufacturing facility. The manufacturing facility...
-
Required information [ The following information applies to the questions displayed below. ] Warnerwoods Company uses a periodic inventory system. It entered into the following purchases and sales...
-
Calculate the number of moles of each ion present in 2.00 10 2 cm 3 of (a) 0.200 M NaCl, (b) 0.350 M K 3 PO 4 , (c) 1.44 M Al(NO 3 ) 3 .
-
How would you prepare 250.0 mL of a 0.350 M NaOH solution from a 6.00 M NaOH stock solution?
-
Imagine a firm wherein, all costs were incurred in the first half of the year and all revenue earned in the second half? You would see a semi-annual income statement for the first half of the year...
-
Construct a 90% confidence interval for the population standard deviation o at Bank B. Bank B 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Jamila Traders has a head office in Nanyuki and an autonomous branch in Thika. The trial balances of the head office and the branch as at 30 September 2014 were as follows: Head office Sh. Sh. Thika...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
ROI analysis using the DuPont model a. Firm A has a margin of 7%, sales of $980,000, and ROI of 19.6%. Calculate the firm's average total assets. b. Firm B has net income of $259,200, turnover of...
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
Find the shortest distance between the following pairs of parallel lines. [x y z]T = [3 0 2]T + t[3 1 0]T [x y z]T = [-1 2 2]T + t[3 1 0]T
-
Hotel Majestic is interested in estimating fixed and variable costs so that the company can make more accurate projections of costs and profit. The hotel is in a resort area that is particularly busy...
-
The pin support is made from a steel rod and has a diameter of 20 mm. Determine the stress components at points C and D and represent the results on a volume element located at each of these points....
-
The pin support is made from a steel rod and has a diameter of 20 mm. Determine the stress components at points A and B and represent the results on a volume element located at each of these points....
-
The uniform sign has a weight of 1500 lb and is supported by the pipe AB, which has an inner radius of 2.75 in. and an outer radius of 3.00 in. If the face of the sign is subjected to a uniform wind...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


