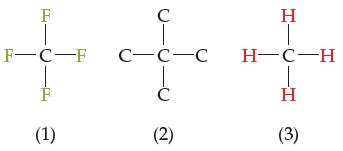
Consider the central carbon atom in each of the three molecules below. (a) One molecule contains a
Question:
Consider the central carbon atom in each of the three molecules below.
(a) One molecule contains a central carbon in its highest possible oxidation state. Which molecule is it, what is its oxidation state, and why is this the highest oxidation state you will ever find a carbon atom in?
(b) One molecule contains a central carbon in its lowest possible oxidation state. Which molecule is it, what is its oxidation state, and why is this the lowest oxidation state you will ever find a carbon atom in?
(c) Which molecule(s) has/have a central carbon atom that satisfies the octet rule?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





