Consider the following process (e represents an electron): (a) Give the full symbol for the starting
Question:
Consider the following process (e– represents an electron):
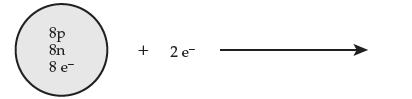
(a) Give the full symbol for the starting atom.
(b) Give the full symbol for the resulting ion.
(c) Is the product a cation or an anion? Explain.
Transcribed Image Text:
8p 8n 8 e + 2 e
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a O b 10 c o Explanation a O indicates a neutral oxygen becuase it has 8 electrons 8 prot...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Dr. Zolar is trying to replicate Solomon Asch's conformity experiments in a collectivist culture. He's not seeing the degree of conformity in the lab that Asch saw. Which reason is most likely is...
-
Consider the case study presented in Section 15.5 involving the Texago Corp. site selection problem. Texago management has tentatively chosen St. Louis as the site of the new refinery. However,...
-
Consider the CuAu crystal structure. It can b e described as a simple cubic lattice with a basis of Cu (0, 0, 0), Cu (1/2, 1/2, 0), Au (1/2, 0, 1/2), and Au (0, 1/2, 1/2). (a) How many atoms of each...
-
A 25,000 kW turbo-generator is supplied with 128,000 kg/h of steam at 2.50 MPa and 400C when developing it rated load. There are actually extracted 10,400 kg h at 0.3 MPa and 8300 kg/h at 0.06 MPa....
-
The needs of service-oriented companies in analyzing financial statements differ from those of product-oriented companies. Why is this true? Give an example of a ratio that is meaningless to a...
-
(a) Suppose K, M: U U are self-adjoint linear functions on an inner product space U. Prove that (k[u], u) = (M[u], u) for all u U if and only if K = M. (b) Explain why this result is false if the...
-
Describe international distribution. LO.1
-
Ian Muse has prepared the following list of statements about the time period assumption. 1. Adjusting entries would not be necessary if a companys life were not divided into artificial time periods....
-
Heels, a shoe manufacturer, is evaluating the costs and benefits of new equipment that would custom fit each pair of athletic shoes. The customer would have his or her foot scanned by digital...
-
Fill in the blanks for each of the atoms below (protons are red, neutrons are blue, and electrons are black): Element Ion (yes or no) Charge of atom Atomic number Mass number ||| |||| ||
-
Give the full symbol for the atom or ion that has 26 protons and 30 neutrons in its nucleus, and 23 electrons outside its nucleus. Also give the number of the group this element is in.
-
For a trip from Boston to Washington, compare the opportunity costs of taking Amtrak and United Airlines. As the time it takes to get through airports has increased, other means of travel have begun...
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
Recall Problem 5.1.11 where metal plate thicknesses are normally distributed with a mean of 4.3 mm and a standard deviation of 0.12 mm. (a) If one metal plate is placed on top of another, what is the...
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
Draw a Lewis dot structure for each of the following atoms: (a) Carbon (b) Oxygen (c) Fluorine (d) Hydrogen (e) Bromine (f) Sulfur (g) Chlorine (h) Iodine
-
Rank the indicated bonds in terms of increasing bond length: . 1 =C .
-
Predict the geometry for the central atom in each of the compounds below: a) NH 3 b) H 3 O + c) BH 4 d) BCl 3 e) BCl 4 f) CCl 4 g) CHCl 3 h) CH 2 Cl 2
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


