Consider the following reaction. It is unbalanced. (a) Draw in another molecule(s) from among those already there
Question:
Consider the following reaction. It is unbalanced.
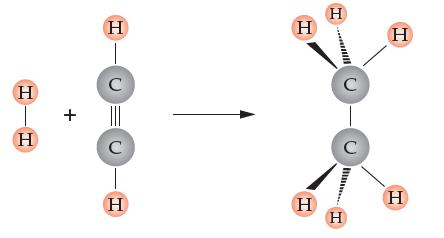
(a) Draw in another molecule(s) from among those already there that completes the reaction.
(b) Write a balanced equation using chemical formulas and balancing coefficients.
(c) Translate the reaction into an English statement using the word moles as many times as you can.
(d) Calculate how many grams of H2 are required to make 10.0 g of product.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





