Consider the ground-state beryllium (Be) and carbon (C) atoms, shown below. (a) Indicate which atom is smaller
Question:
Consider the ground-state beryllium (Be) and carbon (C) atoms, shown below.
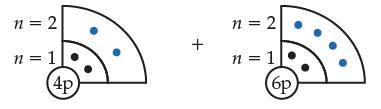
(a) Indicate which atom is smaller and explain why.
(b) Which ground-state atom has valence electrons that look like dumbell-shaped clouds? Explain how you knew this.
Transcribed Image Text:
n = 2 n = 1 4p + n = 2 n = 1 (6p)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a The beryllium atom is smaller than the carbon atom This is because the beryllium atom has a smaller nuclear charge The nuclear charge is the force t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Your home business uses 570 square feet of your 2,850 square foot home. If household expenses for the year were $28,558, how much was alloted to your business? Amount invested
-
Newport Beach Manufacturing Corporation uses a standard cost system that records raw materials at actual cost, records materials price variances at the time that raw materials are issued to work in...
-
In addition to inquiring of individuals among management who are involved in financial reporting positions, such as the CFO and controller, which additional individuals should you consider making...
-
How are changes in the fair value ofan option accounted for in a cash flow hedge? In a fair value hedge? LO9
-
Determinant of Interest Rates The real risk-free rate of interest is 4%. Inflation is expected to be 2% this year and 4% during the next 2 years. Assume that the maturity risk premium is zero. What...
-
The following account balances were drawn from the financial records of Kent Company (KC) as of January 1, Year 5: Assets, $15,000; Liabilities, $4,200; Common Stock, $7,400; and Retained Earnings,...
-
Explain what the following diagram has to say about where one might find lithiums valence electron. 2s
-
Regarding the nth period element whose position is indicated below with an X, which statement(s) are correct? (a) The element is a representative nonmetal. (b) The element is a transition metal. (c)...
-
Find the court decision located at T. C. Summary Opinion 2006-20. a. Which court heard the case? b. Who was the judge(s)? c. Which tax year(s) is in question and in what year was the case decided? d....
-
Tristan Walker of Walker & Company says, "We are only going to design, develop, and test products and services uniquely tailored to our community's needs. I get it. I'm a part of the community we are...
-
Ace Cosmetics Corporation purchased land adjacent to its plant to improve access for trucks making deliveries. Expenditures incurred in purchasing the land were as follows: purchase price, $55,000;...
-
7. At this point you now know information about both the horizontal and the vertical components of the projectile's velocity. In the space below, draw a diagram of the vector components of Vx and...
-
Complete autonomy in how you demonstrate the following criteria. In this module, we talked more about leadership. We discussed the differences between leadership theory which is a well-substantiated...
-
Accustart Ltd. acquired 38% of the common shares of Lecce Ltd. on January 1, 2024, by paying $5.76 million for 144,000 shares. Lecce declared a cash dividend of $0.60 per share in each quarter that...
-
Let a, b, and a - b denote the three edges of a triangle with lengths a, b, and c, respectively. Use Lagrange's Identity together with 2a b = ||a||2 + ||b||2 - ||a - b||2 to prove Heron's Formula for...
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
Use the anharmonic potential function in Figure 18.7 to demonstrate that rotation and vibration are not separable degrees of freedom for large quantum numbers. Figure 18.7 (X)A
-
Conservation of energy requires that the variation of the potential and kinetic energies with the oscillator extension be exactly out of phase. Explain this statement.
-
What is the degeneracy of the energy levels for the rigid rotor in two dimensions? If it is not 1, explain why.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


