Regarding the nth period element whose position is indicated below with an X, which statement(s) are correct?
Question:
Regarding the nth period element whose position is indicated below with an “X”, which statement(s) are correct?
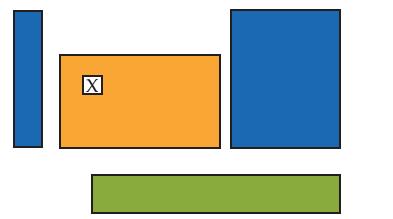
(a) The element is a representative nonmetal.
(b) The element is a transition metal.
(c) The element is in the p block.
(d) The element is in the d block.
(e) The valence electron configuration is ns2(n – 1)d3.
(f) The valence electron configuration is nd5.
(g) The valence electron configuration is (n – 1)d4.
Transcribed Image Text:
X 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The correct statements are c The element is in the p block f The valence electron configuration is nd5 The element X is in the p block because it is in column 5 of the periodic table The p block contains the representative elements so the element X is a representative nonmetal The valence electron configuration of the element X is nd5 because it is in group 5 of the periodic table The incorrect statements are a The element is a representative nonmetal This statement is correct b The element is a transition metal This statement is incorrect because the element X is in the p block not the d block d The element is in the d block This statement is incorrect because the element X is in the p block not the d block e The valence electron configuration is ns2n 1d3 This statement is incorrect because the valence electron configuration of the element X is nd5 not ns2n 1d3 g The valence electron configuration is n ...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Emily Jackson (Social Security number 765-12-4326) and James Stewart (Social Security number 466-74-9932) are partners in a partnership that owns and operates a barber shop. The partnership's first...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Calculate the anion gap for a 58 year old insulin dependent diabetic woman who is admitted to the emergency room in a comatose state Na-135mmol/L K= 3.5 mmol/L CI-102 mmol/L HCO3-= 15mmol/L. Include...
-
Suzy-Q Corporation has established the following standard cost per unit: Materials5.5 lb@$2.20 per lb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $12.10 Labor1.8 hr@$6.25 per...
-
The chapter vignette "Asset Misappropriation Collusion at Koss Corporation" on page 314 highlights the fraud at Koss Corporation, where the principal accounting officer, Sujata ("Sue") Sachdeva,...
-
In what way is the accounting for a foreign currency borrowing more complicated than the account ing for a foreign currency account payable? LO9
-
Consider the problem of constructing (not solving) crossword puzzles:5 fitting words into a rectangular grid. The grid, which is given as part of the problem, specifics which square are blank and...
-
tment for the Data Table ding Work-in-Process Iny ixing Departma per unit amounts to the ne Units Costs OS 0 26,000 54,9259 Paints port-Mixing Dog eptember 30, 2 26,000 54,925 Beginning...
-
Consider the ground-state beryllium (Be) and carbon (C) atoms, shown below. (a) Indicate which atom is smaller and explain why. (b) Which ground-state atom has valence electrons that look like...
-
(a) The following diagram depicts the formation of LiCl. According to the diagram, what are the charges of the lithium cation and the chlorine anion? (b) The diagram yields charges for the ions that...
-
Plaster Inc. received a $0.15-per-share cash dividend on 50,000 shares of Gestalt Corporation common stock, which Plaster Inc. carries as a long-term investment. Assuming that Plaster Inc. uses the...
-
A carload of Hg-ore containing grains of cinnabar (86%Hg by mass; density = 8.19 g/cm3) and grains of basalt (containing no Hg; density=2.84 g/cm3) is to be sampled and analyzed for mercury. The...
-
CMS reviews acute IPPS and long-term care hospital (LTCH) records for payment purposes. Documentation and coding assignment must be accurate and specific. CMS contracts with Medicare Administrative...
-
Problem 2. x3+2x+1 f(x) = = 5-x 8H xx (4 points) Without graphing the function, find the limits lim f(x) and lim f(x) analyt- ically and show your work. Specify if the limits are - or +. (1 point)...
-
For change management, answer the following questions in detail, citing some industry examples: 1. What would you do if your manager requested you change your way of working on a project? 2. What do...
-
1.Sony has just released a new CD recording (okay, not new because we don't buy CDS) but anyway.Here is some cost and price information: CD Disc and Packaging (material and labor) $1.75/CD...
-
Let vectors a, b, and c with common initial point determine a tetrahedron, and let m, n, p, and q be vectors perpendicular to the four faces, pointing outward, and having length equal to the area of...
-
Aztec Furnishings makes hand-crafted furniture for sale in its retail stores. The furniture maker has recently installed a new assembly process, including a new sander and polisher. With this new...
-
For a two-dimensional harmonic oscillator, V (x, y) = k x x 2 + k y y 2 Write an expression for the energy levels of such an oscillator in terms of k x and k y .
-
A gas-phase 1 H 127 I molecule, with a bond length of 160.92 pm, rotates in three dimensional space. a. Calculate the zero point energy associated with this rotation. b. What is the smallest quantum...
-
In this problem you will derive the commutator [lx, ly] = ihlz. a. The angular momentum vector in three dimensions has the form l = il x + jl y + kl z , where the unit vectors in the x, y, and z...
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


