Does the following Bohr model represent a ground or excited state? Explain your answer. 3 Fece CC
Question:
Does the following Bohr model represent a ground or excited state? Explain your answer.
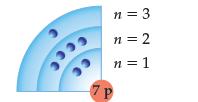
Transcribed Image Text:
3 Fece CC n = 3 n = 2 n = 1 7P
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The Bohr model representation given 7P does not provi...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The first code snippet allows you to simulate the wave function starting the finite difference method either from the left or from the right boundary. If the numeric method is working, we do not...
-
a. Starting with the expression f t 1 for a wave packet, find an expression for the product E t for a photon. b. Interpret your expression. What does it tell you? c. The Bohr model of atomic...
-
The Bohr model of the atom is specified in Problem 9A.8. (a) What features of it are untenable according to quantum mechanics? (b) How does the Bohr ground state differ from the actual ground state?...
-
Reply as to whether you believe the following statements are correct (C) or incorrect (I) concerning PPS sampling. a. The size of a PPS sample is not based on the estimated variation of audited...
-
Depreciation and Cash Flow Ohare Companys only asset as of January 1, 2010, was a limousine. During 2010, only the following three transactions occurred: Services of $100,000 were provided on...
-
Apply the inverse power method of Exercise 10.6.7 to the find the smallest eigenvalue of the matrices in Exercise 10.6.1. In Exercise 10.6.1 Use the power method to find the dominant eigenvalue and...
-
Name the industries that rely the most on franchising to tap foreign markets. LO.1
-
Oregon Forests uses a joint process to manufacture two grades of wood: A and B. During October 2013, the company incurred $ 16,200,000 of joint production cost in producing 27,000,000 board feet of...
-
The Gutchi Company manufactures purses, shaving bags, and backpacks. The construction includes leather and synthetics, leather being the scarce raw material. The production process requires two types...
-
Explain why we construct a Bohr model of the atom by first filling a lower shell to capacity before going to an upper shell.
-
Of what significance is the quantity 2 2 ?
-
What must be true for something to be an economic good?
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
The amount of sugar contained in 1-kg packets is actually normally distributed with a mean of = 1.03 kg and a standard deviation of = 0.014 kg. (a) What proportion of sugar packets are underweight?...
-
we have to compute the letter grades for a course. The data is a collection of student records stored in a file. Each record consists of a name(up to 20 characters), ID (8 characters), the scores of...
-
The mass spectrum of 2-bromopentane shows many fragments. a) One fragment appears at M79. Would you expect a signal at M77 that is equal in height to the M79 peak? Explain. b) A fragment appears at...
-
When treated with a strong base, 2-bromo-2,3-dimethylbutane will undergo an elimination reaction to produce two products. The choice of base (ethoxide vs. tert-butoxide) will determine which of the...
-
Propose a molecular formula that fits the following data. a) A hydrocarbon (C x H y ) with a molecular ion peak at m/z = 66 b) A compound that absorbs IR radiation at 1720 cm -1 and exhibits a...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


