Fluorouracil is a compound administered to cancer patients as a part of chemotherapy. Assign an oxidation state
Question:
Fluorouracil is a compound administered to cancer patients as a part of chemotherapy. Assign an oxidation state to every atom:
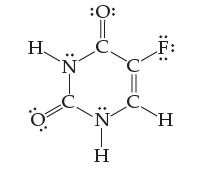
Transcribed Image Text:
H 0 -Z: N :Z-H :: H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The following is the oxidation state assignment for each atom in the molecule fluorouracilC4H3FN2O2 ...View the full answer

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
In methane, CH 4 , (a) Assign an oxidation state to each atom in the molecule. (b) How many electrons does the C atom own by oxidation-state electron bookkeeping? (c) How many more or fewer valence...
-
Consider the organic compound C 3 H 6 O 2 , methyl acetate. (a) What is the problem with using the shortcut method to assign oxidation numbers to the atoms in methyl acetate? (b) The dot diagram of...
-
In chloroform, CHCl 3 , (a) Assign an oxidation state to each atom in the molecule. (b) How many electrons does the C atom own by oxidation-state electron bookkeeping? (c) How many more or fewer...
-
Given the following set of slope staking notes: C Sta 51+00 50+00 49+00 L CX 33.4 F 9.1 33.6. F 10.3 35.4 C 5.5 0.0 F 3.5 R C 3.2 X C 4.1 30.2 0.0 20.0 Bases Base for cut=48 ft Base for fill= 40 ft s...
-
In a recent annual report, eBay Inc. reported that during the year it issued stock of $128 million for acquisitions. How would this be reported on the statement of cash flows?
-
John Smith, age 31, is single and has no dependents. At the beginning of 2017, John started his own excavation business and named it Earth Movers. John lives at 1045 Center Street, Lindon, UT, and...
-
Discuss Paulas (the OD managers) actions from the viewpoint of various leadership and management theories related to roles and responsibilities, organizational transformation, power, organizational...
-
The retained earnings account for Carlitos Inc. shows the following debits and credits. Indicate all entries required to correct the account. What is the corrected amount of retainedearnings?...
-
Question 3 (a) Suppose Amazon.com has offered you free shipping on your next purchase of more than USD35. (i) Identify the type of promotional tool that this offer belongs to. (1 mark) (ii) Briefly...
-
Assign an oxidation state to each carbon in: (a) H 3 CCH 3 (b) H 2 CCH 2 (c) HCCH
-
Tin cans are actually iron plated with tin. What is the advantage of the tin coating?
-
The best placement for the underlined portion would be: F. Where it is now. G. After the word Go. H. After the word firewall. J. At the beginning of the sentence.
-
HOW DO WE CONNECT SAILORS TO THEIR PAST IN ORDER TO TEACH VALUES, HENCE ENHANCING PRIDE IN SERVICE TO OUR COUNTRY?
-
Making this substitution using 12 sin(x) cos(x) dx gives us 12 sin(x) (1-sin(x)) cos(x) dx = 12 sin(x) cos(x) dx-
-
For MNEs In light of the pandemic, do you agree that globalisation is in retreat? Why?
-
How do emergent states such as cohesion, potency, and mental models influence team effectiveness and performance in complex and dynamic environments ?
-
2. How do you feel about the progress IKEA Group has made in implementing this plan? I'm looking for analysis for 2-3 pages with a minimum of 3-4 references for this case. Case study: Sustainability...
-
If L is a linear transformation from V to IV, use mathematical induction to prove that L(a1v1 + a2v2 +...... + anvn) = a1L(V2) + a2L(v2) +.......+ anL(vn)
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
For what point in a pumping system is the NPSH computed? Why?
-
It is desired to operate a pump at 1750 rpm by driving it with a four-pole electric motor. For each of the following conditions, compute the specific speed using Eq. (1317). Then, recommend whether...
-
Compute the specific speed for a pump operating at 3500 rpm delivering 500 gal/min of water at a total head of 100 ft. Compare the result with that of Problem 13.40 and with Fig. 13.52. Flow (m3/h)...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


