Consider the organic compound C 3 H 6 O 2 , methyl acetate. (a) What is the
Question:
Consider the organic compound C3H6O2, methyl acetate.
(a) What is the problem with using the shortcut method to assign oxidation numbers to the atoms in methyl acetate?
(b) The dot diagram of methyl acetate is shown below. Assign an oxidation state to every atom.
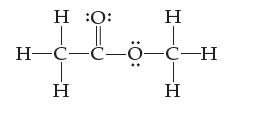
Transcribed Image Text:
Η :Ο: H¬C-C-0-C-H ά Η Η Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
a The problem with using the shortcut method to assign oxidation numbers to the atoms in methyl acet...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The hydroquinone molecule can be converted to the quinone molecule as shown below: You may want to use a combination of shortcut rules and dot diagrams to assign oxidation numbers before answering...
-
Consider the three compounds shown below and then answer the questions that follow: a) Which two compounds are constitutional isomers? b) Which compound contains a nitrogen atom with trigonal...
-
F 6 I 2 O 4 is an interesting compound whose structure is shown below. Complete the dot diagram (add lone pairs) and then determine the oxidation state for every atom in the molecule not using the...
-
I currently have to complete an assignment on normalization anddenormalization, but I'm lost and don't know hat to do! General Instructions You must answer the following questions and create a...
-
Steward Inc. sells a product for $40 per unit. The variable cost is $30 per unit, and fixed costs are $15,000. Determine (a) The break-even point in sales units and (b) The breakeven point in sales...
-
1. In the June 2005 agreement, what consideration did Amber offer in exchange for Fredericks granting her a half-interest in the property? 2. Is love and affection alone consideration for a contract?...
-
Describe what negative unanimity would be.
-
Consider a Solow economy that begins with a capital stock equal to $300 billion, and suppose its steady-state level of capital is $500 billion. To its pleasant surprise, the economy receives a...
-
In the project you will assume the role of the staff accountant for Shipping Inc., and perform Shipping Inc.'s accounting for the transactions that occurred during January 2021. A list of the...
-
What do the terms oxidation and reduction mean with regard to valence electrons?
-
Consider ClO and AlCl 3 . For one of these substances, the halide shortcut rule works. For the other, it does not. (a) Which one does it work for, and why? (b) Why doesnt it work for the other? (c)...
-
What features would you expect to see in computer software that helps with location decisions? Do a survey of relevant packages. How do they work, and what analyses do they do?
-
You have two dashboards in the same workspace named Production and Manufacturing. Your company's Power BI administrator creates the following two dashboard data classifications: Medium Impact (MEDI)...
-
Question 2: Red Rocks Corporation was organized on September 1. Red Rocks encountered the following events during the first month of operations. a. Received $65,000 cash from the investors who...
-
he previous three weeks of data is below for the sales of sheds at SHEDS INC. Calculate the forecast for the next perioud (week 4) using a two period weighted moving average using weights of 3 and 2....
-
/3 3) ST tan(x) - In(cosx) dx What is the value of u? us dulcis) What is the corresponding value of du? du= 1-5mx dx cosx You must show all of your work in the space below to earn full credit. 9/3 So...
-
Please use the file which provides the data to answer the problems 1-3. Problem 1) The time Students entered the classroom of OM 390, Introductory Operations Management, was recorded by the professor...
-
Show that if U and V are subspaces of Rn and U V = {0}, then dim (U + V) = dim U + dim V
-
In Problems, solve each system of equations. x + 2y + 3z = 5 y + 11z = 21 5y + 9z = 13
-
A popular circus act features daredevil motorcycle riders encased in the ???Globe of Death??? (Fig. P5.65), a spherical metal cage of diameter 16 ft.? (a) A rider of mass 65 kg on a 125-cc (95-kg)...
-
An ancient and deadly weapon, a sling consists of two braided cords, each about half an arm???s length long, attached to a leather pocket. The pocket is loaded with a projectile made of lead, carved...
-
A man stands 6.0 ft tall at sea level on the North Pole as shown in Figure P5.62.? (a) What is the difference in the value of g (the gravitational acceleration) between his head and his feet?? (b)...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


