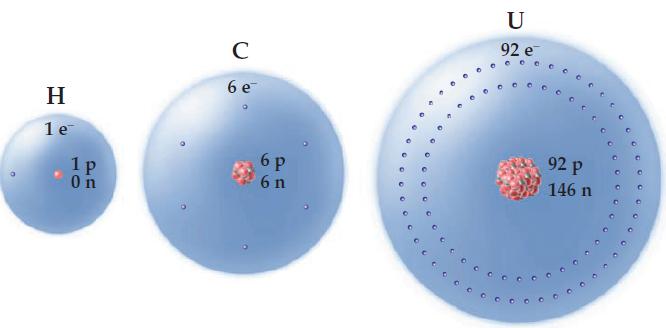
From the figure, what do you think is the rule for determining the number of electrons a
Question:
From the figure, what do you think is the rule for determining the number of electrons a neutral atom contains?
Transcribed Image Text:
H T 1 e 1 p On C бе бр 6 n 0cco U 92 e cccccc 92 р 146 п O G000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
From the figure it is obvious that an atom has as many e...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The financial information provided below is for two companies which operate in similar retail fields, using the same business and accounting policies A. Calculate for each company, ratios that shows...
-
What do you think is McDonalds near-term and long-term potential? What makes you think this?
-
28. The amount of time spent by an employee in the factory is usually recorded on: a. time tickets b. job order cost sheets c. employees' earnings records d. statement of owners'equity 29. The entry...
-
Describe the broad range of talent management efforts that use software applications by going to www.learn.com. Then give some examples of firms that have successfully used these applications.
-
Shortly after being hired as an analyst with Global American Airlines, Kim Williams was asked to prepare a report that focused on passenger ticketing cost. The airline writes most of its own tickets...
-
E14.5. Reverse Engineering with Two-stage Growth Rates (Medium) An analyst develops the following pro forma at the end of 2009 (in millions): 2009A 2010E 2011E Operating income $ 782 $ 868 Net...
-
What is Donna's most effective approach to giving voice to her values? Explain.
-
Use the following financial statements of Precision Co. to complete these requirements . Compute the following ratios as of December 31, 20 23 and 2012 , or for the year ended December 31, 20 23 and...
-
Other things in nature besides chemical properties are periodic. List as many things in nature as you can that are periodic, and include the period for each.
-
An atoms mass number tells you (a) The mass of an atom. (b) How many protons are in the nucleus. (c) How many neutrons are in the nucleus. (d) How many (protons + neutrons) are in the nucleus.
-
What is the difference between setting the bold variable to True, False, or None?
-
Based on the following information, calculate the sustainable growth rate for Kaleb's Welding Supply: Profit margin Capital intensity ratio Debt-equity ratio Net income Dividends 7.5% 0.65 0.60...
-
Waterway Inc. uses LIFO inventory costing. At January 1, 2025, inventory was $216,014 at both cost and market value. At December 31, 2025, the inventory was $283,252 at cost and $262,660 at market...
-
What is the 32-bit version of: 0000 0000 0001 0101
-
1. Let A = 2 1 4 3 Find AT, A-1, (A-1) and (AT)-1. 2. Let A = = [ -1 -1 2 22 (a) Find (AB), BT AT and AT BT. (b) (AB)-1, B-1A-1 and A-B-1. ] 1-5 and B = 1
-
Xavier Ltd. paid out cash dividends at the end of each year as follows: Year Dividend Paid 2018 $250,000 2019 $325,000 2020 $400,000 Assume that Xavier had 100,000 common shares and 5,000, $4,...
-
Determine the rotation angle 6 needed to eliminate the cross-product term in 7x2 + 8xy + y2 = 9. Then obtain the corresponding uv-equation and identify the conic that it represents.
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
First-order decay processes as described in the previous problem can also be applied to a variety of atomic and molecular processes. For example, in aqueous solution the decay of singlet molecular...
-
In a subsequent chapter we will encounter the energy distribution P () = Ae /kT , where P () is the probability of a molecule occupying a given energy state, is the energy of the state, k is a...
-
Assume that the probability of occupying a given energy state is given by the relationship provided in Problem P29.27. In problem 29.27 In a subsequent chapter we will encounter the energy...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


