Give the IUPAC names for: (a) (b) H | HC-H H-C-H |
Question:
Give the IUPAC names for:
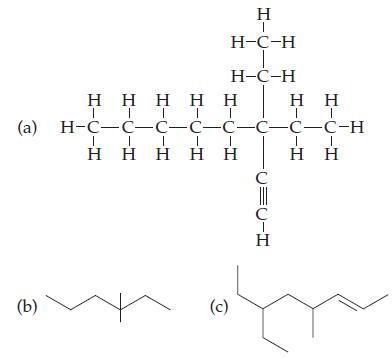
Transcribed Image Text:
(a) (b) H | H¬C-H H-C-H Η Η Η Η Η | | | ΤΙ (c) H-C-C-C-C-C-C-C-C-H | 1 | Η Η Η Η Η C U=U-I C Η H Η | Η Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a 33diethyl1...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Give the IUPAC names for each of the following: (a) (b) (c) (d) (e) (f) CI Cl
-
Give the IUPAC names for each of the following compounds: (a) CH2CH(CH2)5CHCH2 (b) (c) (CH2CH)3CH (d) (e) (f) CH2CCHCHCHCH3 (g) (h) CH3 CHz CH3 HH CI CI H H H3C CH,CH2 CH2CH3
-
Give the IUPAC names for each of the following compounds: (a) (CH3CH2)2C==CHCH3 (b) (CH3CH2)2C==C(CH2CH3)2 (c) (CH3)3CCH==CCl2 (d) (e) (f) (g) , ,
-
Which of the following is not an example of supervised machine learning? O Clustering O Deep learning O Decision Tree Linear Regression
-
In the below figure, a consumer is initially in equilibrium at point C. The consumer??s income is $300, and the budget line through point C is given by $300 = $50X + $100Y. When the consumer is given...
-
Consider the collision between an asteroid and a rocket as discussed in connection with Figure 7.17. We have already calculated the deflection angle that results from the collision with the rocket...
-
Cost and financial accounts are reconciled under (a) Integral system (b) Cost-control accounts system (c) Both a and b (d) None of these
-
The common stock of Escapist Films sells for $25 a share and offers the following payoffs next year: Calculate the expected return and standard deviation of Escapist. All three scenarios are equally...
-
Please help on only one answer! please provide answer to part h) only. please note that 1,015,000 is not correct. thank you! A&R Quality Advisors is a small consulting firm offering quality audits...
-
A functionalized hydrocarbon is a hydrocarbon that: (a) Has many useful functions. (b) Possesses one or more heteroatoms (atoms other than C and H). (c) Is cyclic instead of linear. (d) Has...
-
Make a line drawing for: (a) C=C (b) H-C-C-C-C-C-C-H (c) H -C-H
-
An incinerator burns 120 MT/h of MSW with the formula C285H455O235N4S. How much air is needed to completely combust this waste? A rate of 35% excess air is used during combustion.
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
A police officer is investigating the scene of an accident where two cars collided at an intersection. One car with a mass of 1100 kg moving west had collided with a 1300-kg car moving north. The two...
-
Kenneth Hubbard has prepared the following list of statements about managerial accounting and financial accounting. 1. Financial accounting focuses on providing information to internal users. 2....
-
The energy per unit area per unit time per unit wavelength interval emitted by a blackbody at a temperature T is given by At a specific temperature, the total power radiated per unit area of the...
-
Start with Eq. (13.4) and show that its equivalent to where λ is in meters, T is in kelvins, and the wavelength interval Îλ should be in nanometers. Then I...
-
In the atomic domain, energy is often measured in electronvolts. Arrive at the following expression for the energy of a light-quantum in eV when the wavelength is in nanometers: What is the energy of...
-
Problem 12.6A (Algo) Liquidation of a partnership LO P5 Kendra, Cogley, and Mel share income and loss in a 3.21 ratio (in ratio form: Kendra, 3/6: Cogley, 2/6; and Mel, 1/6), The partners have...
-
Melody Property Limited owns a right to use land together with a building from 2000 to 2046, and the carrying amount of the property was $5 million with a revaluation surplus of $2 million at the end...
-
Famas Llamas has a weighted average cost of capital of 9.1 percent. The companys cost of equity is 12.6 percent, and its cost of debt is 7.2 percent. The tax rate is 25 percent. What is the companys...

Study smarter with the SolutionInn App


